3D Culture Modeling of Metastatic Breast Cancer Cells in Additive Manufactured Scaffolds
- PMID: 35687666
- PMCID: PMC9227707
- DOI: 10.1021/acsami.2c07492
3D Culture Modeling of Metastatic Breast Cancer Cells in Additive Manufactured Scaffolds
Abstract
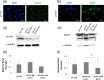
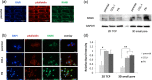
Cancer biology research is increasingly moving toward innovative in vitro 3D culture models, as conventional and current 2D cell cultures fail to resemble in vivo cancer biology. In the current study, porous 3D scaffolds, designed with two different porosities along with 2D tissue culture polystyrene (TCP) plates were used with a model breast cancer human cell line. The 3D engineered system was evaluated for the optimal seeding method (dynamic versus static), adhesion, and proliferation rate of MDA-MB-231 breast cancer cells. The expression profiles of proliferation-, stemness-, and dormancy-associated cancer markers, namely, ki67, lamin A/C, SOX2, Oct3/4, stanniocalcin 1 (STC1), and stanniocalcin 2 (STC2), were evaluated in the 3D cultured cells and compared to the respective profiles of the cells cultured in the conventional 2D TCP. Our data suggested that static seeding was the optimal seeding method with porosity-dependent efficiency. Moreover, cells cultured in 3D scaffolds displayed a more dormant phenotype in comparison to 2D, which was manifested by the lower proliferation rate, reduced ki67 expression, increased lamin A/C expression, and overexpression of STCs. The possible relationship between the cell affinity to different extracellular matrix (ECM) proteins and the RANK expression levels was also addressed after deriving collagen type I (COL-I) and fibronectin (FN) MDA-MB-231 filial cell lines with enhanced capacity to attach to the respective ECM proteins. The new derivatives exhibited a more mesenchymal like phenotype and higher RANK levels in relation to the parental cells, suggesting a relationship between ECM cell affinity and RANK expression. Therefore, the present 3D cell culture model shows that cancer cells on printed scaffolds can work as better representatives in cancer biology and drug screening related studies.
Keywords: breast cancer; scaffolds; three-dimensional bioprinting; tissue engineering; tumor microenvironment.
Conflict of interest statement
The authors declare no competing financial interest.
Figures





Similar articles
-
Enhanced Metastatic Potential in a 3D Tissue Scaffold toward a Comprehensive in Vitro Model for Breast Cancer Metastasis.ACS Appl Mater Interfaces. 2015 Dec 23;7(50):27810-22. doi: 10.1021/acsami.5b09064. Epub 2015 Dec 8. ACS Appl Mater Interfaces. 2015. PMID: 26599258
-
Development and verification of a three-dimensional (3D) breast cancer tumor model composed of circulating tumor cell (CTC) subsets.Mol Biol Rep. 2020 Jan;47(1):97-109. doi: 10.1007/s11033-019-05111-z. Epub 2019 Oct 3. Mol Biol Rep. 2020. PMID: 31583566
-
Collagen-I and fibronectin modified three-dimensional electrospun PLGA scaffolds for long-term in vitro maintenance of functional hepatocytes.Mater Sci Eng C Mater Biol Appl. 2020 Jun;111:110723. doi: 10.1016/j.msec.2020.110723. Epub 2020 Feb 3. Mater Sci Eng C Mater Biol Appl. 2020. PMID: 32279797
-
Advances in biofabrication techniques for collagen-based 3D in vitro culture models for breast cancer research.Mater Sci Eng C Mater Biol Appl. 2021 Mar;122:111944. doi: 10.1016/j.msec.2021.111944. Epub 2021 Feb 5. Mater Sci Eng C Mater Biol Appl. 2021. PMID: 33641930 Review.
-
Advancement of Scaffold-Based 3D Cellular Models in Cancer Tissue Engineering: An Update.Front Oncol. 2021 Oct 25;11:733652. doi: 10.3389/fonc.2021.733652. eCollection 2021. Front Oncol. 2021. PMID: 34760696 Free PMC article. Review.
Cited by
-
Identification and Validation of STC1 Act as a Biomarker for High-Altitude Diseases and Its Pan-Cancer Analysis.Int J Mol Sci. 2024 Aug 21;25(16):9085. doi: 10.3390/ijms25169085. Int J Mol Sci. 2024. PMID: 39201771 Free PMC article.
-
Microvascular network based on the Hilbert curve for nutrient transport in thick tissue.Regen Biomater. 2024 Aug 26;11:rbae094. doi: 10.1093/rb/rbae094. eCollection 2024. Regen Biomater. 2024. PMID: 39350955 Free PMC article.
-
The stromal-tumor amplifying STC1-Notch1 feedforward signal promotes the stemness of hepatocellular carcinoma.J Transl Med. 2023 Mar 31;21(1):236. doi: 10.1186/s12967-023-04085-8. J Transl Med. 2023. PMID: 37004088 Free PMC article.
-
A 3D-Printable Cell Array for In Vitro Breast Cancer Modeling.Int J Mol Sci. 2024 Dec 5;25(23):13068. doi: 10.3390/ijms252313068. Int J Mol Sci. 2024. PMID: 39684779 Free PMC article.
-
Research Progress in the Field of Tumor Model Construction Using Bioprinting: A Review.Int J Nanomedicine. 2024 Jun 27;19:6547-6575. doi: 10.2147/IJN.S460387. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38957180 Free PMC article. Review.
References
-
- Mehta V.; Goel S.; Kabarriti R.; Cole D.; Goldfinger M.; Acuna-Villaorduna A.; Pradhan K.; Thota R.; Reissman S.; Sparano J. A.; et al. Case Fatality Rate of Cancer Patients with COVID-19 in a New York Hospital System. Cancer discovery 2020, 10 (7), 935–941. 10.1158/2159-8290.CD-20-0516. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

