Brain injury environment critically influences the connectivity of transplanted neurons
- PMID: 35687687
- PMCID: PMC9187233
- DOI: 10.1126/sciadv.abg9445
Brain injury environment critically influences the connectivity of transplanted neurons
Abstract
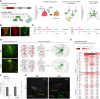
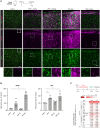
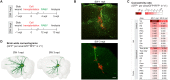
Cell transplantation is a promising approach for the reconstruction of neuronal circuits after brain damage. Transplanted neurons integrate with remarkable specificity into circuitries of the mouse cerebral cortex affected by neuronal ablation. However, it remains unclear how neurons perform in a local environment undergoing reactive gliosis, inflammation, macrophage infiltration, and scar formation, as in traumatic brain injury (TBI). To elucidate this, we transplanted cells from the embryonic mouse cerebral cortex into TBI-injured, inflamed-only, or intact cortex of adult mice. Brain-wide quantitative monosynaptic rabies virus (RABV) tracing unraveled graft inputs from correct regions across the brain in all conditions, with pronounced quantitative differences: scarce in intact and inflamed brain versus exuberant after TBI. In the latter, the initial overshoot is followed by pruning, with only a few input neurons persisting at 3 months. Proteomic profiling identifies candidate molecules for regulation of the synaptic yield, a pivotal parameter to tailor for functional restoration of neuronal circuits.
Figures


Similar articles
-
Selective plasticity of callosal neurons in the adult contralesional cortex following murine traumatic brain injury.Nat Commun. 2022 May 12;13(1):2659. doi: 10.1038/s41467-022-29992-0. Nat Commun. 2022. PMID: 35551446 Free PMC article.
-
Retrograde monosynaptic tracing through an engineered human embryonic stem cell line reveals synaptic inputs from host neurons to grafted cells.Cell Regen. 2019 Mar 1;8(1):1-8. doi: 10.1016/j.cr.2019.01.002. eCollection 2019 Jun. Cell Regen. 2019. PMID: 31205682 Free PMC article.
-
Excessive local host-graft connectivity in aging and amyloid-loaded brain.Sci Adv. 2022 Jun 10;8(23):eabg9287. doi: 10.1126/sciadv.abg9287. Epub 2022 Jun 10. Sci Adv. 2022. PMID: 35687689 Free PMC article.
-
Organization of dopamine and serotonin system: Anatomical and functional mapping of monosynaptic inputs using rabies virus.Pharmacol Biochem Behav. 2018 Nov;174:9-22. doi: 10.1016/j.pbb.2017.05.001. Epub 2017 May 2. Pharmacol Biochem Behav. 2018. PMID: 28476484 Review.
-
Updated Toolbox for Assessing Neuronal Network Reconstruction after Cell Therapy.Bioengineering (Basel). 2024 May 14;11(5):487. doi: 10.3390/bioengineering11050487. Bioengineering (Basel). 2024. PMID: 38790353 Free PMC article. Review.
Cited by
-
COPD basal cells are primed towards secretory to multiciliated cell imbalance driving increased resilience to environmental stressors.Thorax. 2024 May 20;79(6):524-537. doi: 10.1136/thorax-2022-219958. Thorax. 2024. PMID: 38286613 Free PMC article.
-
Emergence of task-related spatiotemporal population dynamics in transplanted neurons.Nat Commun. 2023 Nov 11;14(1):7320. doi: 10.1038/s41467-023-43081-w. Nat Commun. 2023. PMID: 37951968 Free PMC article.
-
Wrapping stem cells with wireless electrical nanopatches for traumatic brain injury therapy.Nat Commun. 2024 Aug 22;15(1):7223. doi: 10.1038/s41467-024-51098-y. Nat Commun. 2024. PMID: 39174514 Free PMC article.
-
Commonly Overlooked Factors in Biocompatibility Studies of Neural Implants.Adv Sci (Weinh). 2023 Feb;10(6):e2205095. doi: 10.1002/advs.202205095. Epub 2023 Jan 3. Adv Sci (Weinh). 2023. PMID: 36596702 Free PMC article. Review.
-
Custom-engineered hydrogels for delivery of human iPSC-derived neurons into the injured cervical spinal cord.Biomaterials. 2024 Mar;305:122400. doi: 10.1016/j.biomaterials.2023.122400. Epub 2023 Nov 17. Biomaterials. 2024. PMID: 38134472 Free PMC article.
References
-
- Barker R. A.; TRANSEURO consortium , Designing stem-cell-based dopamine cell replacement trials for Parkinson’s disease. Nat. Med. 25, 1045–1053 (2019). - PubMed
-
- Espuny-Camacho I., Michelsen K. A., Linaro D., Bilheu A., Acosta-Verdugo S., Herpoel A., Giugliano M., Gaillard A., Vanderhaeghen P., Human pluripotent stem-cell-derived cortical neurons integrate functionally into the lesioned adult murine visual cortex in an area-specific way. Cell Rep. 23, 2732–2743 (2018). - PMC - PubMed
-
- Palma-Tortosa S., Tornero D., Hansen M. G., Monni E., Hajy M., Kartsivadze S., Aktay S., Tsupykov O., Parmar M., Deisseroth K., Skibo G., Lindvall O., Kokaia Z., Activity in grafted human iPS cell–derived cortical neurons integrated in stroke-injured rat brain regulates motor behavior. Proc. Natl. Acad. Sci. U.S.A. 117, 9094–9100 (2020). - PMC - PubMed
-
- Falkner S., Grade S., Dimou L., Conzelmann K. K., Bonhoeffer T., Götz M., Hübener M., Transplanted embryonic neurons integrate into adult neocortical circuits. Nature 539, 248–253 (2016). - PubMed
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

