Sutimlimab in patients with cold agglutinin disease: results of the randomized placebo-controlled phase 3 CADENZA trial
- PMID: 35687757
- PMCID: PMC9437710
- DOI: 10.1182/blood.2021014955
Sutimlimab in patients with cold agglutinin disease: results of the randomized placebo-controlled phase 3 CADENZA trial
Abstract
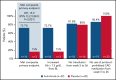
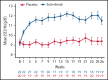
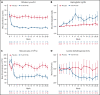
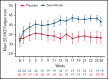
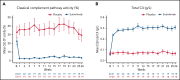
Sutimlimab, a first-in-class humanized immunoglobulin G4 (IgG4) monoclonal antibody that selectively inhibits the classical complement pathway at C1s, rapidly halted hemolysis in the single-arm CARDINAL study in recently transfused patients with cold agglutinin disease (CAD). CADENZA was a 26-week randomized, placebo-controlled phase 3 study to assess safety and efficacy of sutimlimab in patients with CAD without recent (within 6 months prior to enrollment) transfusion history. Forty-two patients with screening hemoglobin ≤10 g/dL, elevated bilirubin, and ≥1 CAD symptom received sutimlimab (n = 22) or placebo (n = 20) on days 0 and 7 and then biweekly. Composite primary endpoint criteria (hemoglobin increase ≥1.5 g/dL at treatment assessment timepoint [mean of weeks 23, 25, 26], avoidance of transfusion, and study-prohibited CAD therapy [weeks 5-26]) were met by 16 patients (73%) on sutimlimab, and 3 patients (15%) on placebo (odds ratio, 15.9 [95% confidence interval, 2.9, 88.0; P < .001]). Sutimlimab, but not placebo, significantly increased mean hemoglobin and FACIT-Fatigue scores at treatment assessment timepoint. Sutimlimab normalized mean bilirubin by week 1. Improvements correlated with near-complete inhibition of the classical complement pathway (2.3% mean activity at week 1) and C4 normalization. Twenty-one (96%) sutimlimab patients and 20 (100%) placebo patients experienced ≥1 treatment-emergent adverse event. Headache, hypertension, rhinitis, Raynaud phenomenon, and acrocyanosis were more frequent with sutimlimab vs placebo, with a difference of ≥3 patients between groups. Three sutimlimab patients discontinued owing to adverse events; no placebo patients discontinued. These data demonstrate that sutimlimab has potential to be an important advancement in the treatment of CAD. This trial was registered at www.clinicaltrials.gov as #NCT03347422.
© 2022 by The American Society of Hematology.
Figures

Comment in
-
A virtuosic CADENZA played by sutimlimab.Blood. 2022 Sep 1;140(9):933-935. doi: 10.1182/blood.2022017284. Blood. 2022. PMID: 36048476 No abstract available.
References
-
- Berentsen S. Complement activation and inhibition in autoimmune hemolytic anemia: focus on cold agglutinin disease. Semin Hematol. 2018;55(3): 141-149. - PubMed
-
- Berentsen S, Ulvestad E, Langholm R, et al. . Primary chronic cold agglutinin disease: a population based clinical study of 86 patients. Haematologica. 2006;91(4): 460-466. - PubMed
-
- Röth A, Fryzek J, Jiang X, et al. . Complement-mediated hemolysis persists year round in patients with cold agglutinin disease. Transfusion. 2022; 62(1):51-59. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

