Rationally Guided Improvement of NOV1 Dioxygenase for the Conversion of Lignin-Derived Isoeugenol to Vanillin
- PMID: 35687874
- PMCID: PMC9851154
- DOI: 10.1021/acs.biochem.2c00168
Rationally Guided Improvement of NOV1 Dioxygenase for the Conversion of Lignin-Derived Isoeugenol to Vanillin
Abstract
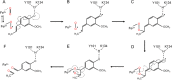
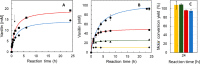
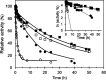
Biocatalysis is a key tool in both green chemistry and biorefinery fields. NOV1 is a dioxygenase that catalyzes the one-step, coenzyme-free oxidation of isoeugenol into vanillin and holds enormous biotechnological potential for the complete valorization of lignin as a sustainable starting material for biobased chemicals, polymers, and materials. This study integrates computational, kinetic, structural, and biophysical approaches to characterize a new NOV1 variant featuring improved activity and stability compared to those of the wild type. The S283F replacement results in a 2-fold increased turnover rate (kcat) for isoeugenol and a 4-fold higher catalytic efficiency (kcat/Km) for molecular oxygen compared to those of the wild type. Furthermore, the variant exhibits a half-life that is 20-fold higher than that of the wild type, which most likely relates to the enhanced stabilization of the iron cofactor in the active site. Molecular dynamics supports this view, revealing that the S283F replacement decreases the optimal pKa and favors conformations of the iron-coordinating histidines compatible with an increased level of binding to iron. Importantly, whole cells containing the S283F variant catalyze the conversion of ≤100 mM isoeugenol to vanillin, yielding >99% molar conversion yields within 24 h. This integrative strategy provided a new enzyme for biotechnological applications and mechanistic insights that will facilitate the future design of robust and efficient biocatalysts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



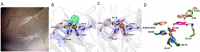


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

