Conjugation with 8-arm PEG and CRM197 enhances the immunogenicity of SARS-CoV-2 ORF8 protein
- PMID: 35687905
- PMCID: PMC9168007
- DOI: 10.1016/j.intimp.2022.108922
Conjugation with 8-arm PEG and CRM197 enhances the immunogenicity of SARS-CoV-2 ORF8 protein
Abstract
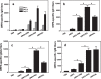
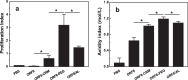
Safe and effective vaccines are urgently needed to combat the COVID-19 pandemic. However, the SARS-CoV-2 variants raise concerns about the effectiveness of vaccines. As a SARS-CoV-2 antigen target, ORF8 strongly inhibits the IFN-β and NF-κB-responsive promoter, and can be potentially used for the development of SARS-CoV-2 vaccine. However, it is necessary to improve the immunogenicity of ORF8 by adjuvants or delivery systems. CRM197 was a carrier protein with the ability to activate T helper cells for antigens. Eight-arm PEG could conjugate multiple antigen molecules in one entity with inherent adjuvant effect. In the present study, ORF8 was conjugated with CRM197 and 8-arm PEG, respectively. The cellular and humoral immune responses to the conjugates (ORF8-CRM and ORF8-PEG) were evaluated in the BALB/c mice. As compared with ORF8-CRM and ORF8 administrated with aluminum adjuvant (ORF8/AL), ORF8-PEG induced a higher ORF8-specific IgG titer (2.6 × 104), higher levels of cytokines (IFN-γ, TNF-α, IFN-β, and IL-5), stronger splenocyte proliferation. Thus, conjugation with 8-arm PEG was an effective method to improve the immune response to ORF8. Moreover, ORF8-PEG did not lead to apparent toxicity to the cardiac, liver and renal functions. ORF8-PEG was expected to act as an effective vaccine to provide the immune protection against SARS-CoV-2.
Keywords: CRM(197); ORF8; SARS-CoV-2; Vaccine; eight-arm PEG.
Copyright © 2022 Elsevier B.V. All rights reserved.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures
Similar articles
-
Conjugation of Zika virus EDIII with CRM197, 8-arm PEG and mannan for development of an effective Zika virus vaccine.Int J Biol Macromol. 2021 Nov 1;190:713-721. doi: 10.1016/j.ijbiomac.2021.08.177. Epub 2021 Aug 30. Int J Biol Macromol. 2021. PMID: 34474053
-
A SARS-CoV-2 nanoparticle vaccine based on chemical conjugation of loxoribine and SpyCatcher/SpyTag.Int J Biol Macromol. 2023 Dec 31;253(Pt 5):127159. doi: 10.1016/j.ijbiomac.2023.127159. Epub 2023 Sep 29. Int J Biol Macromol. 2023. PMID: 37778577
-
Conjugation of the CRM197-inulin conjugate significantly increases the immunogenicity of Mycobacterium tuberculosis CFP10-TB10.4 fusion protein.Bioorg Med Chem. 2017 Nov 1;25(21):5968-5974. doi: 10.1016/j.bmc.2017.09.027. Epub 2017 Sep 19. Bioorg Med Chem. 2017. PMID: 28967465
-
SARS-CoV-2 ORF8: One protein, seemingly one structure, and many functions.Front Immunol. 2022 Oct 24;13:1035559. doi: 10.3389/fimmu.2022.1035559. eCollection 2022. Front Immunol. 2022. PMID: 36353628 Free PMC article. Review.
-
SARS-CoV-2 ORF8 as a Modulator of Cytokine Induction: Evidence and Search for Molecular Mechanisms.Viruses. 2024 Jan 22;16(1):161. doi: 10.3390/v16010161. Viruses. 2024. PMID: 38275971 Free PMC article. Review.
References
-
- Finkel Y., Mizrahi O., Nachshon A., Weingarten-Gabbay S., Morgenstern D., Yahalom-Ronen Y., Tamir H., Achdout H., Stein D., Israeli O., Beth-Din A., Melamed S., Weiss S., Israely T., Paran N., Schwartz M., Stern-Ginossar N. The coding capacity of SARS-CoV-2. Nature. 2021;589(7840):125–130. - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

