Meaningful use of imaging resources to rule out cerebral venous sinus thrombosis after ChAdOx1 COVID-19 vaccination: Evaluation of the AHA diagnostic algorithm with a clinical cohort and a systematic data review
- PMID: 35687921
- PMCID: PMC9167954
- DOI: 10.1016/j.jocn.2022.05.031
Meaningful use of imaging resources to rule out cerebral venous sinus thrombosis after ChAdOx1 COVID-19 vaccination: Evaluation of the AHA diagnostic algorithm with a clinical cohort and a systematic data review
Abstract
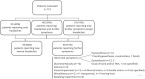
Vaccine-induced immune thrombotic thrombocytopenia (VITT) with cerebral venous thrombosis (CVST) is an improbable (0.0005%), however potentially lethal complication after ChAdOx1 vaccination. On the other hand, headache is among the most frequent side effects of ChAdOx1 (29.3%). In September 2021, the American Heart Association (AHA) suggested a diagnostic workflow to facilitate risk-adapted use of imaging resources for patients with neurological symptoms after ChAdOx1. We aimed to evaluate the AHA workflow in a retrospective patient cohort presenting at four primary care hospitals in Germany for neurological complaints after ChAdOx1. Scientific literature was screened for case reports of VITT with CVST after ChAdOx1, published until September 1st, 2021. One-hundred-thirteen consecutive patients (77 female, mean age 38.7 +/- 11.9 years) were evaluated at our institutes, including one case of VITT with CVST. Further 228 case reports of VITT with CVST are published in recent literature, which share thrombocytopenia (225/227 reported) and elevated d-dimer levels (100/101 reported). The AHA workflow would have recognized all VITT cases with CVST (100% sensitivity), the number needed to diagnose (NND) was 1:113. Initial evaluation of thrombocytopenia or elevated d-dimer levels would have lowered the NND to 1:68, without cost of sensitivity. Hence, we suggest that in case of normal thrombocyte and d-dimer levels, the access to further diagnostics should be limited by the established clinical considerations regardless of vaccination history.
Keywords: American Heart Association; Headache; Intracranial thrombosis; Meaningful use; Vaccines.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: DM is on the speaker’s bureau of Philips Healthcare. AM is a consultant for Stryker, Perflow, Phenox and Balt. SF is a consultant for Microvention. MS received personal honoraria from Alexion, Biogen, Gilead, Roche, and Sanofi. All other authors have nothing to disclose.
Figures



References
-
- COVID-19 Vaccines | FDA n.d. https://www.fda.gov/emergency-preparedness-and-response/coronavirus-dise... (accessed March 18, 2021).
-
- COVID-19 vaccines: authorised | European Medicines Agency n.d. https://www.ema.europa.eu/en/human-regulatory/overview/public-health-thr... (accessed March 18, 2021).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

