The Persistence of Sex Bias in High-Impact Clinical Research
- PMID: 35687931
- PMCID: PMC11953574
- DOI: 10.1016/j.jss.2022.04.077
The Persistence of Sex Bias in High-Impact Clinical Research
Abstract
Introduction: Sex bias is present in clinical research resulting in disparities in the treatment of women. Our objective was to identify the prevalence of sex inclusiveness of participants in human clinical trials after the passage of National Institutes of Health (NIH) and United States Congress policies in 2015 and 2016 to increase female enrollment in clinical research.
Methods: We performed an observational analysis of data from registered clinical trials published in three high-impact biomedical journals from January 1, 2015 to December 31, 2019.
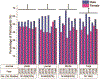
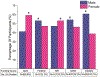
Results: One thousand four hundred and forty two manuscripts with 4,765,783 human subjects were included for analysis. Significantly more males (56%) than females (44%) were included in all three journals (P < 0.0001). Sex matching ≥ 80% was found in 24.6% of publications. Industry funded 43.7% of all studies enrolling significantly more males than females (60.8% versus 39.2%, P < 0.0001). NIH funded 10.2% of studies enrolling significantly more females than males (52.7% versus 47.3%, P < 0.0001). North America and Europe contributed 82.6% of the studies with each enrolling significantly more males than females (P < 0.0001). The United States was the country contributing the most studies (36.1%), enrolling significantly more males than females (55.5% versus 45.5%, P < 0.0001). Cardiovascular disease was the subject area of the most manuscripts among medical specialties (19%), enrolling significantly more males than females (64.9% versus 35.1%, P < 0.0001). Studies analyzed by clinical trial phase, type, trial, and allocation enrolled significantly more males than females (P < 0.0001).
Conclusions: Sex bias remains prevalent in human clinical research trials. Improvements have been made in NIH-funded clinical trials; however, this constitutes a small percentage of overall studies.
Keywords: Clinical research; Disparity; Sex bias; Women.
Published by Elsevier Inc.
Conflict of interest statement
Figures
References
-
- Pinn VW. Sex and gender factors in medical studies: implications for health and clinical practice. JAMA. Jan 22-29 2003;289(4):397–400 - PubMed
-
- Institute of Medicine. Women and Health Research: Ethical and Legal Issues of Including Women in Clinical Studies: Volume I. The National Academic Press; 1994. - PubMed
-
- Evaluation of Sex-Specific Data in Medical Device Clinical Studies - Guidance for Industry and Food and Drug Administration Staff. U.S Food and Drug Administration. 2014. Accessed June 6th, 2021. https://www.fda.gov/regulatory-information/search-fda-guidance-documents...
-
- Steering Committee of the Physicians’ Health Study Research G. Final report on the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. Jul 20 1989;321(3):129–35. - PubMed
-
- Steering Committee of the Physicians’ Health Study Research G. Preliminary report: Findings from the aspirin component of the ongoing Physicians’ Health Study. N Engl J Med. Jan 28 1988;318(4):262–4. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

