Who are the real high-risk patients with pathological T2N0M0 non-small-cell lung cancer that can benefit from adjuvant chemotherapy?
- PMID: 35688064
- PMCID: PMC9184557
- DOI: 10.1016/j.esmoop.2022.100508
Who are the real high-risk patients with pathological T2N0M0 non-small-cell lung cancer that can benefit from adjuvant chemotherapy?
Abstract
Background: The benefit of adjuvant chemotherapy (ACT) in pathological T2N0M0 non-small-cell lung cancer (NSCLC) patients is not clear.
Methods: One thousand and fifty pathological T2N0M0 NSCLC patients were included and divided into two groups: with and without ACT. A propensity score matching analysis was carried out to minimize selection bias. The significance of ACT in high-risk patients was further analyzed. The Kaplan-Meier method and Cox proportional hazards model were used to assess the impact of ACT on the overall survival (OS), disease-free survival (DFS), and cancer-specific survival.
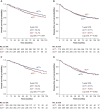
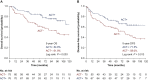
Results: For the entire cohort, 31.9% (335/1050) of patients received ACT. After propensity score matching, 325 pairs of patients were matched. OS and DFS were comparable between groups in the original or matched cohort, which was confirmed by the multivariate analysis (all P > 0.05). In high-risk patients, the data suggest that ACT could improve OS and DFS only in patients with tumours >4 cm (OS: P = 0.003; DFS: P = 0.013). ACT could significantly improve the 5-year OS in patients with wild-type epidermal growth factor receptor (EGFR) (P = 0.022). ACT, however, could not improve cancer-specific survival in any subgroup, including patients with tumours >4 cm or wild-type EGFR (all P > 0.05). For patients with other high-risk factors, ACT failed to benefit patients in long-term outcomes.
Conclusions: In resected pT2N0M0 NSCLC patients, those with tumours >4 cm and wild-type EGFR are real high-risk patients and could gain survival benefit from ACT. Further prospective study is needed to confirm the definition.
Keywords: T2N0M0; adjuvant chemotherapy; non-small cell lung cancer; survival.
Copyright © 2022 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Disclosure None declared.
Figures
References
-
- National Comprehensive Cancer Network . NCCN; 2021. NCCN guideline for non-small cell lung cancer. Version 5.
-
- Strauss G.M., Herndon J.E., II, Maddaus M.A., et al. Adjuvant paclitaxel plus carboplatin compared with observation in stage IB non-small-cell lung cancer: CALGB 9633 with the Cancer and Leukemia Group B, Radiation Therapy Oncology Group, and North Central Cancer Treatment Group Study Groups. J Clin Oncol. 2008;26:5043–5051. - PMC - PubMed
-
- Waller D., Peake M.D., Stephens R.J., et al. Chemotherapy for patients with non-small cell lung cancer: the surgical setting of the Big Lung Trial. Eur J Cardiothorac Surg. 2004;26:173–182. - PubMed
-
- Arriagada R., Bergman B., Dunant A., et al. Cisplatin-based adjuvant chemotherapy in patients with completely resected non-small-cell lung cancer. N Engl J Med. 2004;350:351–360. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

