Cerebrovascular activity is a major factor in the cerebrospinal fluid flow dynamics
- PMID: 35688316
- PMCID: PMC9271599
- DOI: 10.1016/j.neuroimage.2022.119362
Cerebrovascular activity is a major factor in the cerebrospinal fluid flow dynamics
Abstract
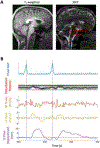
Cerebrospinal fluid (CSF) provides physical protection to the central nervous system as well as an essential homeostatic environment for the normal functioning of neurons. Additionally, it has been proposed that the pulsatile movement of CSF may assist in glymphatic clearance of brain metabolic waste products implicated in neurodegeneration. In awake humans, CSF flow dynamics are thought to be driven primarily by cerebral blood volume fluctuations resulting from a number of mechanisms, including a passive vascular response to blood pressure variations associated with cardiac and respiratory cycles. Recent research has shown that mechanisms that rely on the action of vascular smooth muscle cells ("cerebrovascular activity") such as neuronal activity, changes in intravascular CO2, and autonomic activation from the brainstem, may lead to CSF pulsations as well. Nevertheless, the relative contribution of these mechanisms to CSF flow remains unclear. To investigate this further, we developed an MRI approach capable of disentangling and quantifying CSF flow components of different time scales associated with these mechanisms. This approach was evaluated on human control subjects (n = 12) performing intermittent voluntary deep inspirations, by determining peak flow velocities and displaced volumes between these mechanisms in the fourth ventricle. We found that peak flow velocities were similar between the different mechanisms, while displaced volumes per cycle were about a magnitude larger for deep inspirations. CSF flow velocity peaked at around 10.4 s (range 7.1-14.8 s, n = 12) following deep inspiration, consistent with known cerebrovascular activation delays for this autonomic challenge. These findings point to an important role of cerebrovascular activity in the genesis of CSF pulsations. Other regulatory triggers for cerebral blood flow such as autonomic arousal and orthostatic challenges may create major CSF pulsatile movement as well. Future quantitative comparison of these and possibly additional types of CSF pulsations with the proposed approach may help clarify the conditions that affect CSF flow dynamics.
Keywords: Balanced SSFP MRI; CBF regulation; CSF flow velocity; CSF pulsations; Cerebrovascular activity; Respiratory modulation.
Published by Elsevier Inc.
Conflict of interest statement
Declaration of Competing Interest None.
Figures




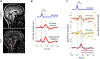
References
-
- Balédent O, Gondry-Jouet C, Meyer M-E, De Marco G, Le Gars D, Henry-Feugeas M-C, Idy-Peretti I, 2004. Relationship Between Cerebrospinal Fluid and Blood Dynamics in Healthy Volunteers and Patients with Communicating Hydrocephalus. Investigative Radiology 39, 45–55. 10.1097/01.rli.0000100892.87214.49 - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

