Approach for reporting master protocol study designs on ClinicalTrials.gov: qualitative analysis
- PMID: 35688481
- PMCID: PMC9186156
- DOI: 10.1136/bmj-2021-067745
Approach for reporting master protocol study designs on ClinicalTrials.gov: qualitative analysis
Abstract
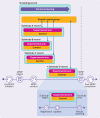
Objective: To describe an approach for reporting master protocol research programs (MPRPs) that is consistent with existing good reporting practices and that uses structured information to convey the overall master protocol and design of each substudy.
Design: Qualitative analysis.
Data sources: ClinicalTrials.gov trial registry.
Main outcome measures: Established goals and related practices of the trial reporting system were outlined, examples and key characteristics of MPRPs were reviewed, and specific challenges in registering and reporting summary results to databases designed for traditional clinical trial designs that rely on a model of one study per protocol were identified.
Results: A reporting approach is proposed that accommodates the complex study design of MPRPs and their results. This approach involves the use of separate registration records for each substudy within one MPRP protocol (with potential exceptions noted).
Conclusions: How the proposed approach allows for clear, descriptive, structured information about each substudy's prespecified design and supports timely reporting of results after completion of each substudy is described and illustrated. Although the focus is on reporting to ClinicalTrials.gov, the approach supports broader application across trial registries and results databases. This paper is intended to stimulate further discussion of this approach among stakeholders, build awareness about the need to improve reporting of MPRPs, and encourage harmonization across trial registries globally.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at https://www.icmje.org/disclosure-of-interest/ and declare: supported, in part, by the National Center for Biotechnology Information and the Intramural Research Program of the National Cancer Institute for the submitted work; RJW, HDD, and TT received support from the National Center for Biotechnology Information of the National Library of Medicine, National Institutes of Health; SDC, DL, GAS, and SAP received support from the Intramural Research Program of the National Cancer Institute, National Institutes of Health; and DAZ received personal fees from ClinicalTrials.gov (and provided technical consultation to ClinicalTrials.gov outside the submitted work); no financial relationships with any organizations that might have an interest in the submitted work in the previous three years; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- US Food and Drug Administration. COVID-19: Master protocols evaluating drugs and biological products for treatment or prevention: Guidance for industry [final guidance for industry]. 2021 May. https://www.fda.gov/regulatory-information/search-fda-guidance-documents....
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
