Targeting of GSDMD sensitizes HCC to anti-PD-1 by activating cGAS pathway and downregulating PD-L1 expression
- PMID: 35688553
- PMCID: PMC9189836
- DOI: 10.1136/jitc-2022-004763
Targeting of GSDMD sensitizes HCC to anti-PD-1 by activating cGAS pathway and downregulating PD-L1 expression
Abstract
Background: Gasdermin D (GSDMD) is well known as a downstream of inflammasomes. However, the roles of GSDMD itself in hepatocellular carcinoma (HCC) remain unclear.
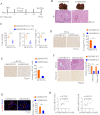
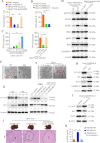
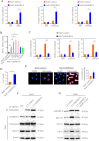
Methods: Two independent cohorts of patients with HCC were analyzed to evaluate the pathological relevance of GSDMD/pTBK1/PD-L1. GSDMD knockout (GSDMD-/-) mice, Alb-Cre mice administered with an adeno-associated virus (AAV) vector that expressed the gasdermin-N domain (AAV9-FLEX-GSDMD-N) and their wild-type littermates were used to induce hepatocarcinogenesis or metastatic HCC. Combination of anti-programmed cell death protein-1 (PD-1) and GSDMD inhibitor dimethyl fumarate (DMF) was used to test for improved therapeutic efficacy. RNA sequencing was used to explore the mechanisms how GSDMD promoted HCC progression.
Results: The expression of GSDMD and GSDMD-N was upregulated in HCC tissues or metastatic HCC tissues and positive GSDMD expression indicated grim prognosis. Diethylnitrosamine/carbon tetrachloride or thioacetamide-treated GSDMD-/- mice exhibited decreased liver tumors. In contrast, AAV9-FLEX-GSDMD-N promoted hepatocarcinogenesis. RNA sequencing manifested that knockout of GSDMD impacted the cyclic GMP-AMP synthase (cGAS) pathway and immune-associated pathway. GSDMD damped cGAS activation by promoting autophagy via outputting potassium (K+) and promoted programmed death ligand-1 (PD-L1) expression by histone deacetylases/signal transducer and activator of transcription 1 (STAT1)-induced transactivation of PD-L1 via importing calcium (Ca2+). High Mobility Group Box 1/toll-like receptor 4 (TLR4)/caspase-1 pathway contributed to the overexpression and cleavage of GSDMD. Anti-PD-1 combining with DMF largely impaired HCC progression and metastasis.
Conclusions: Targeting GSDMD could promote expression of interferons through inactivation of cGAS pathway and downregulated the PD-L1 expression. Therefore, combined anti-PD-1 and GSDMD inhibitor might serve as an effective treatment option for patients with HCC with GSDMD upregulation.
Keywords: CD8-Positive T-Lymphocytes; Immunotherapy; Inflammation; Liver Neoplasms.
© Author(s) (or their employer(s)) 2022. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
