Parallel ventral hippocampus-lateral septum pathways differentially regulate approach-avoidance conflict
- PMID: 35688838
- PMCID: PMC9187740
- DOI: 10.1038/s41467-022-31082-0
Parallel ventral hippocampus-lateral septum pathways differentially regulate approach-avoidance conflict
Abstract
The ability to resolve an approach-avoidance conflict is critical to adaptive behavior. The ventral CA3 (vCA3) and CA1 (vCA1) subfields of the ventral hippocampus (vHPC) have been shown to facilitate avoidance and approach behavior, respectively, in the face of motivational conflict, but the neural circuits by which this subfield-specific regulation is implemented is unknown. We demonstrate that two distinct pathways from these subfields to lateral septum (LS) contribute to this divergent control. In Long-Evans rats, chemogenetic inhibition of the vCA3- LS caudodorsal (cd) pathway potentiated approach towards a learned conflict-eliciting stimulus, while inhibition of the vCA1-LS rostroventral (rv) pathway potentiated approach non-specifically. Additionally, vCA3-LScd inhibited animals were less hesitant to explore food during environmental uncertainty, while the vCA1- LSrv inhibited animals took longer to initiate food exploration. These findings suggest that the vHPC influences multiple behavioral systems via differential projections to the LS, which in turn send inhibitory projections to motivational centres of the brain.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures
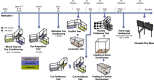
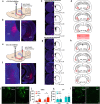


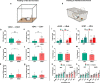
References
-
- Fricke K, Vogel S. How interindividual differences shape approach-avoidance behavior: relating self-report and diagnostic measures of interindividual differences to behavioral measurements of approach and avoidance. Neurosci. Biobehav. Rev. 2020;111:30–56. doi: 10.1016/j.neubiorev.2020.01.008. - DOI - PubMed
-
- Gray, J. A. & McNaughton, N. The Neuropsychology of Anxiety: An Enquiry into the Functions of the Septo-Hippocampal System (Oxford University Press, 2000). 10.1017/S0140525X00013170.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

