Key Modulators of the Stress Granule Response TIA1, TDP-43, and G3BP1 Are Altered by Polyglutamine-Expanded ATXN7
- PMID: 35689166
- PMCID: PMC9363381
- DOI: 10.1007/s12035-022-02888-2
Key Modulators of the Stress Granule Response TIA1, TDP-43, and G3BP1 Are Altered by Polyglutamine-Expanded ATXN7
Abstract
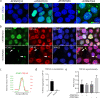
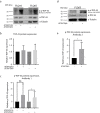
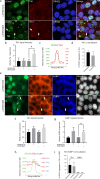
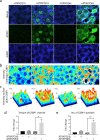
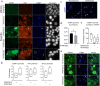
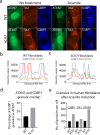
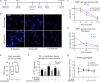
Spinocerebellar ataxia type 7 (SCA7) and other polyglutamine (polyQ) diseases are caused by expansions of polyQ repeats in disease-specific proteins. Aggregation of the polyQ proteins resulting in various forms of cellular stress, that could induce the stress granule (SG) response, is believed to be a common pathological mechanism in these disorders. SGs can contribute to cell survival but have also been suggested to exacerbate disease pathology by seeding protein aggregation. In this study, we show that two SG-related proteins, TDP-43 and TIA1, are sequestered into the aggregates formed by polyQ-expanded ATXN7 in SCA7 cells. Interestingly, mutant ATXN7 also localises to induced SGs, and this association altered the shape of the SGs. In spite of this, neither the ability to induce nor to disassemble SGs, in response to arsenite stress induction or relief, was affected in SCA7 cells. Moreover, we could not observe any change in the number of ATXN7 aggregates per cell following SG induction, although a small, non-significant, increase in total aggregated ATXN7 material could be detected using filter trap. However, mutant ATXN7 expression in itself increased the speckling of the SG-nucleating protein G3BP1 and the SG response. Taken together, our results indicate that the SG response is induced, and although some key modulators of SGs show altered behaviour, the dynamics of SGs appear normal in the presence of mutant ATXN7.
Keywords: G3BP1; Neurodegeneration; Spinocerebellar ataxia type 7; Stress granules; TDP-43; TIA1.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Palhan VB, Chen S, Peng G-H, Tjernberg A, Gamper AM, Fan Y, Chait BT, La Spada AR, Roeder RG. Polyglutamine-expanded ataxin-7 inhibits STAGA histone acetyltransferase activity to produce retinal degeneration. Proc Natl Acad Sci USA. 2005;102(24):8472–8477. doi: 10.1073/pnas.0503505102. - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

