Systematically exploring repurposing effects of antihypertensives
- PMID: 35689299
- PMCID: PMC9545793
- DOI: 10.1002/pds.5491
Systematically exploring repurposing effects of antihypertensives
Abstract
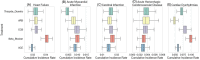
With availability of voluminous sets of observational data, an empirical paradigm to screen for drug repurposing opportunities (i.e., beneficial effects of drugs on nonindicated outcomes) is feasible. In this article, we use a linked claims and electronic health record database to comprehensively explore repurposing effects of antihypertensive drugs. We follow a target trial emulation framework for causal inference to emulate randomized controlled trials estimating confounding adjusted effects of antihypertensives on each of 262 outcomes of interest. We then fit hierarchical models to the results as a form of postprocessing to account for multiple comparisons and to sift through the results in a principled way. Our motivation is twofold. We seek both to surface genuinely intriguing drug repurposing opportunities and to elucidate through a real application some study design decisions and potential biases that arise in this context.
Keywords: antihypertensives; causal inference; drug repurposing; hierarchical models.
© 2022 The Authors. Pharmacoepidemiology and Drug Safety published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- Cushman WC, Ford CE, Cutler JA, et al. Success and predictors of blood pressure control in diverse North American settings: the antihypertensive and lipid‐lowering treatment to prevent heart attack trial (ALLHAT). J Clin Hypertens. 2002;4(6):393‐404. doi: 10.1111/j.1524-6175.2002.02045.x - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

