Management of Cerebral Venous Thrombosis Due to Adenoviral COVID-19 Vaccination
- PMID: 35689346
- PMCID: PMC9349982
- DOI: 10.1002/ana.26431
Management of Cerebral Venous Thrombosis Due to Adenoviral COVID-19 Vaccination
Abstract
Objective: Cerebral venous thrombosis (CVT) caused by vaccine-induced immune thrombotic thrombocytopenia (VITT) is a rare adverse effect of adenovirus-based severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2) vaccines. In March 2021, after autoimmune pathogenesis of VITT was discovered, treatment recommendations were developed. These comprised immunomodulation, non-heparin anticoagulants, and avoidance of platelet transfusion. The aim of this study was to evaluate adherence to these recommendations and its association with mortality.
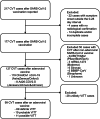
Methods: We used data from an international prospective registry of patients with CVT after the adenovirus-based SARS-CoV-2 vaccination. We analyzed possible, probable, or definite VITT-CVT cases included until January 18, 2022. Immunomodulation entailed administration of intravenous immunoglobulins and/or plasmapheresis.
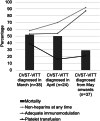
Results: Ninety-nine patients with VITT-CVT from 71 hospitals in 17 countries were analyzed. Five of 38 (13%), 11 of 24 (46%), and 28 of 37 (76%) of the patients diagnosed in March, April, and from May onward, respectively, were treated in-line with VITT recommendations (p < 0.001). Overall, treatment according to recommendations had no statistically significant influence on mortality (14/44 [32%] vs 29/55 [52%], adjusted odds ratio [OR] = 0.43, 95% confidence interval [CI] = 0.16-1.19). However, patients who received immunomodulation had lower mortality (19/65 [29%] vs 24/34 [70%], adjusted OR = 0.19, 95% CI = 0.06-0.58). Treatment with non-heparin anticoagulants instead of heparins was not associated with lower mortality (17/51 [33%] vs 13/35 [37%], adjusted OR = 0.70, 95% CI = 0.24-2.04). Mortality was also not significantly influenced by platelet transfusion (17/27 [63%] vs 26/72 [36%], adjusted OR = 2.19, 95% CI = 0.74-6.54).
Conclusions: In patients with VITT-CVT, adherence to VITT treatment recommendations improved over time. Immunomodulation seems crucial for reducing mortality of VITT-CVT. ANN NEUROL 2022;92:562-573.
© 2022 The Authors. Annals of Neurology published by Wiley Periodicals LLC on behalf of American Neurological Association.
Conflict of interest statement
The authors declare no conflicts of interest related to this manuscript.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

