Adiposity-associated atrial fibrillation: molecular determinants, mechanisms, and clinical significance
- PMID: 35689487
- PMCID: PMC10409902
- DOI: 10.1093/cvr/cvac093
Adiposity-associated atrial fibrillation: molecular determinants, mechanisms, and clinical significance
Abstract
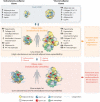
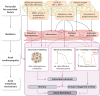
Obesity is an important contributing factor to the pathophysiology of atrial fibrillation (AF) and its complications by causing systemic changes, such as altered haemodynamic, increased sympathetic tone, and low-grade chronic inflammatory state. In addition, adipose tissue is a metabolically active organ that comprises various types of fat deposits with discrete composition and localization that show distinct functions. Fatty tissue differentially affects the evolution of AF, with highly secretory active visceral fat surrounding the heart generally having a more potent influence than the rather inert subcutaneous fat. A variety of proinflammatory, profibrotic, and vasoconstrictive mediators are secreted by adipose tissue, particularly originating from cardiac fat, that promote atrial remodelling and increase the susceptibility to AF. In this review, we address the role of obesity-related factors and in particular specific adipose tissue depots in driving AF risk. We discuss the distinct effects of key secreted adipokines from different adipose tissue depots and their participation in cardiac remodelling. The possible mechanistic basis and molecular determinants of adiposity-related AF are discussed, and finally, we highlight important gaps in current knowledge, areas requiring future investigation, and implications for clinical management.
Keywords: Adipokines; Atrial fibrillation; Epicardial adipose tissue; NLRP3 inflammasome; Obesity; Subcutaneous adipose tissue; Visceral adipose tissue.
© The Author(s) 2022. Published by Oxford University Press on behalf of the European Society of Cardiology. All rights reserved. For permissions, please email: journals.permissions@oup.com.
Conflict of interest statement
Conflict of interest: A.S., N.L., I.A.-T., T.J., D.L., S.N., J.H., and A.F.: none. M.G. received grant from Pfizer and Specialized Research Fellowship (Club 30 of PCS) grant. D.D. is a member of the Scientific Advisory Boards of Omeicos Therapeutics GmbH and Acesion Pharma.
Figures


References
-
- Powell-Wiley TM, Poirier P, Burke LE, Despres JP, Gordon-Larsen P, Lavie CJ, Lear SA, Ndumele CE, Neeland IJ, Sanders P, St-Onge MP; American Heart Association Council on Lifestyle and Cardiometabolic Health; Council on Cardiovascular and Stroke Nursing; Council on Clinical Cardiology; Council on Epidemiology and Prevention; and Stroke Council . Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation 2021;143:e984–e1010. - PMC - PubMed
-
- Schoettl T, Fischer IP, Ussar S. Heterogeneity of adipose tissue in development and metabolic function. J Exp Biol 2018;221:jeb162958. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

