A ferrocene-containing nucleoside analogue targets DNA replication in pancreatic cancer cells
- PMID: 35689667
- PMCID: PMC9320222
- DOI: 10.1093/mtomcs/mfac041
A ferrocene-containing nucleoside analogue targets DNA replication in pancreatic cancer cells
Abstract
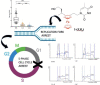
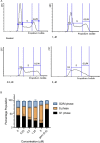
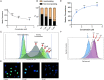
Pancreatic ductal adenocarcinoma (PDAC) is a disease that remains refractory to existing treatments including the nucleoside analogue gemcitabine. In the current study we demonstrate that an organometallic nucleoside analogue, the ferronucleoside 1-(S,Rp), is cytotoxic in a panel of PDAC cell lines including gemcitabine-resistant MIAPaCa2, with IC50 values comparable to cisplatin. Biochemical studies show that the mechanism of action is inhibition of DNA replication, S-phase cell cycle arrest and stalling of DNA-replication forks, which were directly observed at single molecule resolution by DNA-fibre fluorography. In agreement with this, transcriptional changes following treatment with 1-(S,Rp) include activation of three of the four genes (HUS1, RAD1, RAD17) of the 9-1-1 check point complex clamp and two of the three genes (MRE11, NBN) that form the MRN complex as well as activation of multiple downstream targets. Furthermore, there was evidence of phosphorylation of checkpoint kinases 1 and 2 as well as RPA1 and gamma H2AX, all of which are considered biochemical markers of replication stress. Studies in p53-deficient cell lines showed activation of CDKN1A (p21) and GADD45A by 1-(S,Rp) was at least partially independent of p53. In conclusion, because of its potency and activity in gemcitabine-resistant cells, 1-(S,Rp) is a promising candidate molecule for development of new treatments for PDAC.
Keywords: DNA replication; Ferrocene; nucleoside analogue; pancreatic cancer; replication fork arrest.
© The Author(s) 2022. Published by Oxford University Press.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
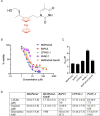
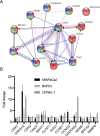
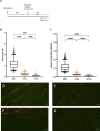
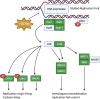
 activation ,
activation ,  inhibition,
inhibition,  binding,
binding,  catalysis,
catalysis,  phenotype,
phenotype,  posttranslational modification,
posttranslational modification,  reaction and
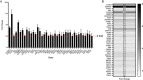
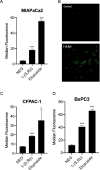
reaction and  transcriptional regulation. (B) Quantification of fold changes as assessed by qPCR in MIAPaCa2, BxPC3 and CFPAC-1 cells following treatment with 1-(S,Rp) (10 μM, 24 h). The red dotted line represents 2-fold increase compared to untreated control. The results represent the mean of three independent biological experiments (±SD, n = 3).
transcriptional regulation. (B) Quantification of fold changes as assessed by qPCR in MIAPaCa2, BxPC3 and CFPAC-1 cells following treatment with 1-(S,Rp) (10 μM, 24 h). The red dotted line represents 2-fold increase compared to untreated control. The results represent the mean of three independent biological experiments (±SD, n = 3).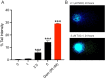
References
-
- Allemani C., Matsuda T., Di Carlo V., Harewood R., Matz M., Niksic M., Bonaventure A., Valkov M., Johnson C. J., Esteve J., Ogunbiyi O. J., Azevedo E. S. G., Chen W. Q., Eser S., Engholm G., Stiller C. A., Monnereau A., Woods R. R., Visser O., Lim G. H., Aitken J., Weir H. K., Coleman M. P., C W. Group, Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries, The Lancet, 2018, 391 (10125), 1023–1075. - PMC - PubMed
-
- Conroy T., Desseigne F., Ychou M., Bouche O., Guimbaud R., Becouarn Y., Adenis A., Raoul J. L., Gourgou-Bourgade S., de la Fouchardiere C., Bennouna J., Bachet J. B., Khemissa-Akouz F., Pere-Verge D., Delbaldo C., Assenat E., Chauffert B., Michel P., Montoto-Grillot C., Ducreux M., Unicanc G. T. D., Intergrp P., FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer, N. Engl. J. Med., 2011, 364 (19), 1817–1825. - PubMed
-
- Pacheco-Barcia V., France T., Zogopoulos G., Bouganim N., Donnay O., Alcindor T., Solis R. M., Guo K., Martin E., Colomer R., Asselah J., Gemcitabine plus nab-paclitaxel versus modified FOLFIRINOX as first line chemotherapy in metastatic pancreatic cancer: a comparison of toxicity and survival, Ann. Oncol., 2018, 29 (Suppl 5), v46.
-
- Williet N., Saint A., Pointet A. L., Tougeron D., Pernot S., Pozet A., Bechade D., Trouilloud I., Lourenco N., Hautefeuille V., Locher C., Desrame J., Artru P., Thirot Bidault A., Le Roy B., Pezet D., Phelip J. M., Taieb J., Folfirinox versus Gemcitabine/Nab-Paclitaxel as first-line therapy in patients with metastatic pancreatic cancer: a comparative propensity score study, Therap. Adv. Gastroenterol., 2019, 12, 175628481987866. - PMC - PubMed
-
- Cho I., Kang H., Jo J., Lee H., Chung M., Park J., Park S., Song S., Park M., An C., Jung S., Bang S., FOLFIRINOX versus gemcitabine plus Nab-Paclitaxel for treatment of metastatic pancreatic cancer: a single-center cohort study, Ann. Oncol., 2018, 29 (Suppl 5), v45.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

