Antagonistic Effect of Plant Growth-Promoting Fungi Against Fusarium Wilt Disease in Tomato: In vitro and In vivo Study
- PMID: 35689755
- PMCID: PMC9587074
- DOI: 10.1007/s12010-022-03975-9
Antagonistic Effect of Plant Growth-Promoting Fungi Against Fusarium Wilt Disease in Tomato: In vitro and In vivo Study
Abstract
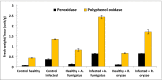
Fusarium wilt is considered one of the most destructive diseases for tomato plants. The novelty of this work was to investigate the antifungal and plant growth-promoting capabilities of some plant growth-promoting fungi (PGPF). Plant growth-promoting fungi (PGPF) improved the plant health and control plant infections. In this study, two fungal strains as PGPF were isolated and identified as Aspergillus fumigatus and Rhizopus oryzae using molecular method. The extracts of A. fumigatus and R. oryzae exhibited promising antifungal activity against F. oxysporum in vitro. Moreover, antagonistic effect of A. fumigatus and R. oryzae against F. oxysporum causing tomato wilt disease was evaluated in vivo. Disease severity and growth markers were recorded and in vitro antagonistic activity assay of the isolated A. fumigatus and R. oryzae against Fusarium oxysporum was measured. Physiological markers of defense in plant as response to stimulate systemic resistance (SR) were recorded. Our results indicated that A. fumigatus and R. oryzae decreased the percentage of disease severity by 12.5 and 37.5%, respectively. In addition, they exhibited relatively high protection percentage of 86.35 and 59.06% respectively. Fusarium wilt was declined the growth parameters, photosynthetic pigments, total soluble carbohydrate, and total soluble protein, whereas content of free proline, total phenols, and the activity of antioxidant enzymes activity increased under infection. Moreover, application of A. fumigatus and R. oryzae on infected plants successfully recovered the loss of morphological traits, photosynthetic pigment total carbohydrates, and total soluble proteins in comparison to infected control plants. PGPF strains in both non-infected and infected plants showed several responses in number and density of peroxidase (POD) and polyphenol oxidase (PPO) isozymes.
Keywords: Antifungal activity; Biological control; Fusarium oxysporum; Induction of systemic resistance; Phytopathology; Plant growth-promoting fungi.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures







References
-
- Wang L, et al. Effects of exogenous nitric oxide on growth and transcriptional expression of antioxidant enzyme mRNA in tomato seedlings under copper stress. Acta Horticulturae Sinica. 2010;37(1):47–52.
-
- O’Connell S, et al. High tunnel and field production of organic heirloom tomatoes: Yield, fruit quality, disease, and microclimate. HortScience. 2012;47(9):1283–1290.
-
- Ramakrishna W, Yadav R, Li K. Plant growth promoting bacteria in agriculture: Two sides of a coin. Applied Soil Ecology. 2019;138:10–18.
-
- Faheed FA, Abd-Elaah GA, Mazen A. Alleviation of disease effect on tomato plants by heat shock and salicylic acid infected with Alternaria solani. International Journal of Agriculture and Biology. 2005;7:783–789.
-
- Selim ME, El-Gammal NA. Role of fusaric acid mycotoxin in pathogensis process of tomato wilt disease caused by Fusarium oxysporum. Journal of Bioprocessing & Biotechniques. 2015;5(10):1.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

