Independent validation of stromal uPA in ABCSG-08: Level 1b evidence for the prognostic value of uPA immunohistochemistry
- PMID: 35689881
- PMCID: PMC9190055
- DOI: 10.1016/j.breast.2022.05.003
Independent validation of stromal uPA in ABCSG-08: Level 1b evidence for the prognostic value of uPA immunohistochemistry
Abstract
Purpose: To validate the prognostic role of urokinase-type plasminogen-activator (uPA) and plasminogen activator inhibitor type-1 (PAI-1) protein expression in FFPE archived tumor samples when assessed by immunohistochemistry.
Patients and methods: Fresh-frozen, paraffin-embedded (FFPE) samples from 303 postmenopausal women with hormone receptor-positive, early breast cancer were investigated. The patients had received 5 years of endocrine therapy in the prospectively randomized ABCSG-8 trial. Immunohistochemistry for stromal uPA and PAI-1 protein expression was correlated with distant recurrence-free survival (DRFS) and overall survival (OS).
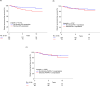
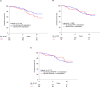
Results: We detected stromal uPA in 132 of 297 tumors (44.4%) and stromal PAI-1 expression in 74 out of 299 samples (24.7%). Co-expression of uPA and PAI-1 was present in 48 of 294 (16.3%) cases. Neither uPA nor PAI-1 expression was associated with tumor size, age, nodal status, grading, or quantitative receptor status. Patients whose tumor stroma expressed uPA protein had a significantly shorter DRFS (adjusted HR for relapse: 2.78; 95% CI 1.31-5.93; p = 0.008 Cox regression analysis) than women without uPA expression. No such association was seen for PAI-1 and the uPA/PAI1 ratio. After a median follow-up of 5.6 years, women with uPA-positive tumors demonstrated significantly shorter DRFS (93.3% vs. 84.8%; p < 0.013 log-rank test), and tended to have a worse OS (83.0% vs. 77.3%; p = 0.106) compared to women with uPA negative tumors.
Conclusion: This independent validation in archived tumor samples from a large prospective randomized trial confirms the clinical utility of stromal uPA evaluation by immunohistochemistry. This provides level 1b evidence for the prognostic role of stromal uPA in women with endocrine-responsive early breast cancer.
Keywords: ABCSG 8; Adjuvant endocrine therapy; Breast cancer; Plasminogen activator Inhibitor-1 (PAI-1); Urokinase plasminogen activator (uPA).
Copyright © 2022 The Authors. Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
All authors have completed and submitted the Declaration of Interest form. Christian Singer reports having received research grants from AstraZeneca, Novartis, Roche, and Amgen, and personal fees/travel support from Amgen, AstraZeneca, EliLilly, Pfizer, Novartis, Roche. Zsuzsanna Bago-Horvath reports congress travel support from Daichii Sankyo, being in the Advisory Board at Roche, MSD. Michael Gnant reports personal fees/travel support from Amgen, DaiichiSankyo, AstraZeneca, EliLilly, LifeBrain, Nanostring, Novartis, PierreFabre, MSD; an immediate family member is employed by Sandoz.
Martin Filipits has received honoraria from Astra Zeneca, Biomedica, Biorad, Boehringer Ingelheim, Eli Lilly, Merck, Novartis, and Pfizer.
Following authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper: Stephan Jahn, Margaretha Rudas, Florian Fitzal, Luca Abete and Farid Moinfar.
Figures
References
-
- Ferraris G.M., Sidenius N. Urokinase plasminogen activator receptor: a functional integrator of extracellular proteolysis, cell adhesion, and signal transduction. Semin Thromb Hemost. 2013;39:347–355. - PubMed
-
- Binder B.R., Mihaly J., Prager G.W. uPAR-uPA-PAI-1 interactions and signaling: a vascular biologist's view. Thromb Haemostasis. 2007;97:336–342. - PubMed
-
- Jiang W.G., Sanders A.J., Katoh M., Ungefroren H., Gieseler F., Prince M., et al. Tissue invasion and metastasis: molecular, biological and clinical perspectives. Semin Cancer Biol. 2015;35(Suppl):S244–S275. - PubMed
-
- Han B., Nakamura M., Mori I., Nakamura Y., Kakudo K. Urokinase-type plasminogen activator system and breast cancer (Review) Oncol Rep. 2005;14:105–112. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

