Persistent cortical and white matter inflammation after therapeutic hypothermia for ischemia in near-term fetal sheep
- PMID: 35690757
- PMCID: PMC9188214
- DOI: 10.1186/s12974-022-02499-7
Persistent cortical and white matter inflammation after therapeutic hypothermia for ischemia in near-term fetal sheep
Abstract
Background: Therapeutic hypothermia significantly improves outcomes after moderate-severe hypoxic-ischemic encephalopathy (HIE), but it is partially effective. Although hypothermia is consistently associated with reduced microgliosis, it is still unclear whether it normalizes microglial morphology and phenotype.
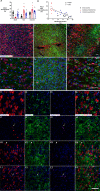
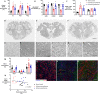
Methods: Near-term fetal sheep (n = 24) were randomized to sham control, ischemia-normothermia, or ischemia-hypothermia. Brain sections were immunohistochemically labeled to assess neurons, microglia and their interactions with neurons, astrocytes, myelination, and gitter cells (microglia with cytoplasmic lipid granules) 7 days after cerebral ischemia. Lesions were defined as areas with complete loss of cells. RNAscope® was used to assess microglial phenotype markers CD86 and CD206.
Results: Ischemia-normothermia was associated with severe loss of neurons and myelin (p < 0.05), with extensive lesions, astrogliosis and microgliosis with a high proportion of gitter cells (p < 0.05). Microglial wrapping of neurons was present in both the ischemia groups. Hypothermia improved neuronal survival, suppressed lesions, gitter cells and gliosis (p < 0.05), and attenuated the reduction of myelin area fraction. The "M1" marker CD86 and "M2" marker CD206 were upregulated after ischemia. Hypothermia partially suppressed CD86 in the cortex only (p < 0.05), but did not affect CD206.
Conclusions: Hypothermia prevented lesions after cerebral ischemia, but only partially suppressed microglial wrapping and M1 marker expression. These data support the hypothesis that persistent upregulation of injurious microglial activity may contribute to partial neuroprotection after hypothermia, and that immunomodulation after rewarming may be an important therapeutic target.
Keywords: Electroencephalogram; Gitter cells; Hypoxia-ischemia; Microglial phenotype; Neuroinflammation; Therapeutic hypothermia.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures








References
-
- Jacobs S, Hunt R, Tarnow-Mordi W, Inder T, Davis P. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database Syst Rev. 2003:CD003311. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

