Reciprocal regulation of NRF2 by autophagy and ubiquitin-proteasome modulates vascular endothelial injury induced by copper oxide nanoparticles
- PMID: 35690781
- PMCID: PMC9188091
- DOI: 10.1186/s12951-022-01486-7
Reciprocal regulation of NRF2 by autophagy and ubiquitin-proteasome modulates vascular endothelial injury induced by copper oxide nanoparticles
Abstract
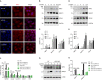
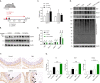
NRF2 is the key antioxidant molecule to maintain redox homeostasis, however the intrinsic mechanisms of NRF2 activation in the context of nanoparticles (NPs) exposure remain unclear. In this study, we revealed that copper oxide NPs (CuONPs) exposure activated NRF2 pathway in vascular endothelial cells. NRF2 knockout remarkably aggravated oxidative stress, which were remarkably mitigated by ROS scavenger. We also demonstrated that KEAP1 (the negative regulator of NRF2) was not primarily involved in NRF2 activation in that KEAP1 knockdown did not significantly affect CuONPs-induced NRF2 activation. Notably, we demonstrated that autophagy promoted NRF2 activation as evidenced by that ATG5 knockout or autophagy inhibitors significantly blocked NRF2 pathway. Mechanically, CuONPs disturbed ubiquitin-proteasome pathway and consequently inhibited the proteasome-dependent degradation of NRF2. However, autophagy deficiency reciprocally promoted proteasome activity, leading to the acceleration of degradation of NRF2 via ubiquitin-proteasome pathway. In addition, the notion that the reciprocal regulation of NRF2 by autophagy and ubiquitin-proteasome was further proven in a CuONPs pulmonary exposure mice model. Together, this study uncovers a novel regulatory mechanism of NRF2 activation by protein degradation machineries in response to CuONPs exposure, which opens a novel intriguing scenario to uncover therapeutic strategies against NPs-induced vascular injury and disease.
Keywords: Autophagy; CuONPs; NRF2; Ubiquitin–proteasome system; Vascular injury.
© 2022. The Author(s).
Conflict of interest statement
The authors declare no conflict of interest.
Figures





References
-
- Holm A, Goodman ED, Stenlid JH, Aitbekova A, Zelaya R, Diroll BT, Johnston-Peck AC, Kao KC, Frank CW, Pettersson LGM, Cargnello M. Nanoscale spatial distribution of supported nanoparticles controls activity and stability in powder catalysts for CO oxidation and photocatalytic H2 evolution. J Am Chem Soc. 2020;142:14481–14494. doi: 10.1021/jacs.0c03842. - DOI - PMC - PubMed
-
- Peter A, Mihaly-Cozmuta L, Mihaly-Cozmuta A, Nicula C, Ziemkowska W, Basiak D, Danciu V, Vulpoi A, Baia L, Falup A, Craciun G, Ciric A, Begea M, Kiss C, Vatuiu D. Changes in the microbiological and chemical characteristics of white bread during storage in paper packages modified with Ag/TiO2-SiO2, Ag/N-TiO2 or Au/TiO2. Food Chem. 2016;197:790–798. doi: 10.1016/j.foodchem.2015.11.048. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

