Neuroprotective effects of ex vivo-expanded regulatory T cells on trimethyltin-induced neurodegeneration in mice
- PMID: 35690816
- PMCID: PMC9188044
- DOI: 10.1186/s12974-022-02512-z
Neuroprotective effects of ex vivo-expanded regulatory T cells on trimethyltin-induced neurodegeneration in mice
Abstract
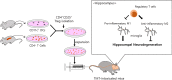
Background: Trimethyltin (TMT) is a potent neurotoxicant that leads to hippocampal neurodegeneration. Regulatory T cells (Tregs) play an important role in maintaining the immune balance in the central nervous system (CNS), but their activities are impaired in neurodegenerative diseases. In this study, we aimed to determine whether adoptive transfer of Tregs, as a living drug, ameliorates hippocampal neurodegeneration in TMT-intoxicated mice.
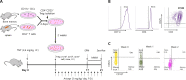
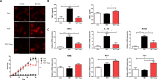
Methods: CD4+CD25+ Tregs were expanded in vitro and adoptively transferred to TMT-treated mice. First, we explored the effects of Tregs on behavioral deficits using the Morris water maze and elevated plus maze tests. Biomarkers related to memory formation, such as cAMP response element-binding protein (CREB), protein kinase C (PKC), neuronal nuclear protein (NeuN), nerve growth factor (NGF), and ionized calcium binding adaptor molecule 1 (Iba1) in the hippocampus were examined by immunohistochemistry after killing the mouse. To investigate the neuroinflammatory responses, the polarization status of microglia was examined in vivo and in vitro using real-time reverse transcription polymerase chain reaction (rtPCR) and Enzyme-linked immunosorbent assay (ELISA). Additionally, the inhibitory effects of Tregs on TMT-induced microglial activation were examined using time-lapse live imaging in vitro with an activation-specific fluorescence probe, CDr20.
Results: Adoptive transfer of Tregs improved spatial learning and memory functions and reduced anxiety in TMT-intoxicated mice. Additionally, adoptive transfer of Tregs reduced neuronal loss and recovered the expression of neurogenesis enhancing molecules in the hippocampi of TMT-intoxicated mice. In particular, Tregs inhibited microglial activation and pro-inflammatory cytokine release in the hippocampi of TMT-intoxicated mice. The inhibitory effects of TMT were also confirmed via in vitro live time-lapse imaging in a Treg/microglia co-culture system.
Conclusions: These data suggest that adoptive transfer of Tregs ameliorates disease progression in TMT-induced neurodegeneration by promoting neurogenesis and modulating microglial activation and polarization.
Keywords: Cell therapy; Hippocampal neurodegeneration; Microglia; Regulatory T cells; Trimethyltin.
© 2022. The Author(s).
Conflict of interest statement
The authors declare that there were no commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




Similar articles
-
Delayed treatment with TGF-β1 associated neuroprotection in trimethyltin-induced hippocampal neurodegeneration.Neurosci Lett. 2025 Mar 15;852:138182. doi: 10.1016/j.neulet.2025.138182. Epub 2025 Mar 4. Neurosci Lett. 2025. PMID: 40049360
-
Neuroprotective effect of Geijigadaehwang-tang against trimethyltin-induced hippocampal neurodegeneration: An in vitro and in vivo study.J Ethnopharmacol. 2022 Oct 5;296:115451. doi: 10.1016/j.jep.2022.115451. Epub 2022 Jun 17. J Ethnopharmacol. 2022. PMID: 35724744
-
Myricetin alleviates learning and memory deficits in trimethyltin Alzheimer's phenotype via attenuating hippocampal endoplasmic reticulum stress and regulating inflammation and oxidative stress.Brain Res Bull. 2025 Jul;227:111382. doi: 10.1016/j.brainresbull.2025.111382. Epub 2025 May 16. Brain Res Bull. 2025. PMID: 40383238
-
Neuroprotective strategies in hippocampal neurodegeneration induced by the neurotoxicant trimethyltin.Neurochem Res. 2013 Feb;38(2):240-53. doi: 10.1007/s11064-012-0932-9. Epub 2012 Nov 25. Neurochem Res. 2013. PMID: 23179590 Review.
-
Trimethyltin-induced hippocampal degeneration as a tool to investigate neurodegenerative processes.Neurochem Int. 2011 Jun;58(7):729-38. doi: 10.1016/j.neuint.2011.03.009. Epub 2011 Mar 22. Neurochem Int. 2011. PMID: 21414367 Review.
Cited by
-
Could immunotherapy and regulatory T cells be used therapeutically to slow the progression of Alzheimer's disease?Brain Commun. 2025 Feb 25;7(2):fcaf092. doi: 10.1093/braincomms/fcaf092. eCollection 2025. Brain Commun. 2025. PMID: 40078868 Free PMC article. Review.
-
The interactive effects of different exercises and hawthorn consumption on the pain threshold of TMT-induced Alzheimer male rats.J Physiol Sci. 2024 Jul 16;74(1):36. doi: 10.1186/s12576-024-00925-4. J Physiol Sci. 2024. PMID: 39014320 Free PMC article.
-
Indications for an antidepressive effect of thymosin alpha-1 in a small open-label proof of concept study in common variable immune deficiency patients with depression.Brain Behav Immun Health. 2025 Jan 2;43:100934. doi: 10.1016/j.bbih.2024.100934. eCollection 2025 Feb. Brain Behav Immun Health. 2025. PMID: 39867848 Free PMC article.
-
Anti-inflammatory effect of grounding mat on trimethyltin-induced neurotoxicity rats.J Exerc Rehabil. 2025 Feb 28;21(1):10-15. doi: 10.12965/jer.2448680.340. eCollection 2025 Feb. J Exerc Rehabil. 2025. PMID: 40083832 Free PMC article.
-
Beta-sitosterol mitigates cognitive deficit and hippocampal neurodegeneration in mice with trimethyltin-induced toxicity.Exp Anim. 2024 Oct 23;73(4):433-445. doi: 10.1538/expanim.24-0021. Epub 2024 Jun 28. Exp Anim. 2024. PMID: 38945945 Free PMC article.
References
-
- Ishida N, Akaike M, Tsutsumi S, Kanai H, Masui A, Sadamatsu M, et al. Trimethyltin syndrome as a hippocampal degeneration model: temporal changes and neurochemical features of seizure susceptibility and learning impairment. Neuroscience. 1997;81(4):1183–1191. doi: 10.1016/S0306-4522(97)00220-0. - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

