Novel App knock-in mouse model shows key features of amyloid pathology and reveals profound metabolic dysregulation of microglia
- PMID: 35690868
- PMCID: PMC9188195
- DOI: 10.1186/s13024-022-00547-7
Novel App knock-in mouse model shows key features of amyloid pathology and reveals profound metabolic dysregulation of microglia
Abstract
Background: Genetic mutations underlying familial Alzheimer's disease (AD) were identified decades ago, but the field is still in search of transformative therapies for patients. While mouse models based on overexpression of mutated transgenes have yielded key insights in mechanisms of disease, those models are subject to artifacts, including random genetic integration of the transgene, ectopic expression and non-physiological protein levels. The genetic engineering of novel mouse models using knock-in approaches addresses some of those limitations. With mounting evidence of the role played by microglia in AD, high-dimensional approaches to phenotype microglia in those models are critical to refine our understanding of the immune response in the brain.
Methods: We engineered a novel App knock-in mouse model (AppSAA) using homologous recombination to introduce three disease-causing coding mutations (Swedish, Arctic and Austrian) to the mouse App gene. Amyloid-β pathology, neurodegeneration, glial responses, brain metabolism and behavioral phenotypes were characterized in heterozygous and homozygous AppSAA mice at different ages in brain and/ or biofluids. Wild type littermate mice were used as experimental controls. We used in situ imaging technologies to define the whole-brain distribution of amyloid plaques and compare it to other AD mouse models and human brain pathology. To further explore the microglial response to AD relevant pathology, we isolated microglia with fibrillar Aβ content from the brain and performed transcriptomics and metabolomics analyses and in vivo brain imaging to measure energy metabolism and microglial response. Finally, we also characterized the mice in various behavioral assays.
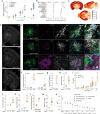
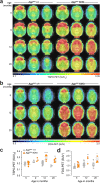
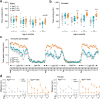
Results: Leveraging multi-omics approaches, we discovered profound alteration of diverse lipids and metabolites as well as an exacerbated disease-associated transcriptomic response in microglia with high intracellular Aβ content. The AppSAA knock-in mouse model recapitulates key pathological features of AD such as a progressive accumulation of parenchymal amyloid plaques and vascular amyloid deposits, altered astroglial and microglial responses and elevation of CSF markers of neurodegeneration. Those observations were associated with increased TSPO and FDG-PET brain signals and a hyperactivity phenotype as the animals aged.
Discussion: Our findings demonstrate that fibrillar Aβ in microglia is associated with lipid dyshomeostasis consistent with lysosomal dysfunction and foam cell phenotypes as well as profound immuno-metabolic perturbations, opening new avenues to further investigate metabolic pathways at play in microglia responding to AD-relevant pathogenesis. The in-depth characterization of pathological hallmarks of AD in this novel and open-access mouse model should serve as a resource for the scientific community to investigate disease-relevant biology.
Keywords: Astrogliosis; Lipid dyshomeostasis; Neuritic plaques; Neurodegeneration; Phagocytic microglia; Vascular amyloid.
© 2022. The Author(s).
Conflict of interest statement
D.X., S.L., T.S., M.C., J.S., E.T., J.D., M.P., N.E.K, J.Y.Z., S.L.D., T.K.E., C.C.L., S.D., C.H., H.H., R.C., E.Y., H.S., S.T.M., R.L., K.L., R.G.T., K.S.L., J.W.L., G.D.P. and P.E.S are paid employees and shareholders of Denali Therapeutic Inc. J.D.W. and J.A.H are currently employed by Cajal Neuroscience.
Figures





References
-
- Scearce-Levie K, Sanchez PE, Lewcock JW. Leveraging preclinical models for the development of Alzheimer disease therapeutics. Nat Rev Drug Discov. 2020;19(7):447–462. - PubMed
-
- Gotz J, Bodea LG, Goedert M. Rodent models for Alzheimer disease. Nat Rev Neurosci. 2018;19(10):583–598. - PubMed
-
- Myers A, McGonigle P. Overview of Transgenic Mouse Models for Alzheimer's Disease. Curr Protoc Neurosci. 2019;89(1):e81. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

