The paradox of radiation and T cells in tumors
- PMID: 35691060
- PMCID: PMC9194456
- DOI: 10.1016/j.neo.2022.100808
The paradox of radiation and T cells in tumors
Abstract
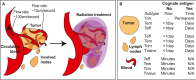
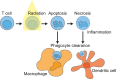
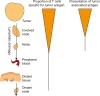
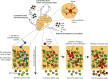
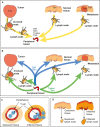
In this review we consider what appears to be a paradox in immunotherapies based around radiation therapy. The paradox is based on three main points. 1. That T cells are needed for radiation's efficacy; 2. That tumor-specific T cells are enriched in the field of treatment; and 3. That radiation kills T cells in the treatment field. We discuss evidence of the effect of radiation on T cells in the field given their ongoing movement in and out of tissues and the tumor, and how the movement of T cells impacts the treated primary tumor and untreated distant metastases. Given this evidence, we revisit the paradox to understand how the extraordinary efficacy of radiation and immunity in preclinical models is dependent on this radiation sensitive cell.
Copyright © 2022. Published by Elsevier Inc.
Figures
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

