Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine in children aged 6-17 years: a preliminary report of COV006, a phase 2 single-blind, randomised, controlled trial
- PMID: 35691324
- PMCID: PMC9183219
- DOI: 10.1016/S0140-6736(22)00770-X
Safety and immunogenicity of the ChAdOx1 nCoV-19 (AZD1222) vaccine in children aged 6-17 years: a preliminary report of COV006, a phase 2 single-blind, randomised, controlled trial
Erratum in
-
Department of Error.Lancet. 2022 Jul 2;400(10345):24. doi: 10.1016/S0140-6736(22)01208-9. Lancet. 2022. PMID: 35780788 Free PMC article. No abstract available.
Abstract
Background: Vaccination of children and young people against SARS-CoV-2 is recommended in some countries. Scarce data have been published on immune responses induced by COVID-19 vaccines in people younger than 18 years compared with the same data that are available in adults.
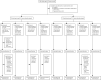
Methods: COV006 is a phase 2, single-blind, randomised, controlled trial of ChAdOx1 nCoV-19 (AZD1222) in children and adolescents at four trial sites in the UK. Healthy participants aged 6-17 years, who did not have a history of chronic respiratory conditions, laboratory-confirmed COVID-19, or previously received capsular group B meningococcal vaccine (the control), were randomly assigned to four groups (4:1:4:1) to receive two intramuscular doses of 5 × 1010 viral particles of ChAdOx1 nCoV-19 or control, 28 days or 84 days apart. Participants, clinical investigators, and the laboratory team were masked to treatment allocation. Study groups were stratified by age, and participants aged 12-17 years were enrolled before those aged 6-11 years. Due to the restrictions in the use of ChAdOx1 nCoV-19 in people younger than 30 years that were introduced during the study, only participants aged 12-17 years who were randomly assigned to the 28-day interval group had received their vaccinations at the intended interval (day 28). The remaining participants received their second dose at day 112. The primary outcome was assessment of safety and tolerability in the safety population, which included all participants who received at least one dose of the study drug. The secondary outcome was immunogenicity, which was assessed in participants who were seronegative to the nucleocapsid protein at baseline and received both prime and boost vaccine. This study is registered with ISRCTN (15638344).
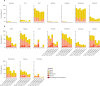
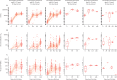
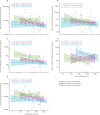
Findings: Between Feb 15 and April 2, 2021, 262 participants (150 [57%] participants aged 12-17 years and 112 [43%] aged 6-11 years; due to the change in the UK vaccination policy, the study terminated recruitment of the younger age group before the planned number of participants had been enrolled) were randomly assigned to receive vaccination with two doses of either ChAdOx1 nCoV-19 (n=211 [n=105 at day 28 and n=106 at day 84]) or control (n=51 [n=26 at day 28 and n=25 at day 84]). One participant in the ChAdOx1 nCoV-19 day 28 group in the younger age bracket withdrew their consent before receiving a first dose. Of the participants who received ChAdOx1 nCoV-19, 169 (80%) of 210 participants reported at least one solicited local or systemic adverse event up to 7 days following the first dose, and 146 (76%) of 193 participants following the second dose. No serious adverse events related to ChAdOx1 nCoV-19 administration were recorded by the data cutoff date on Oct 28, 2021. Of the participants who received at least one dose of ChAdOx1 nCoV-19, there were 128 unsolicited adverse events up to 28 days after vaccination reported by 83 (40%) of 210 participants. One participant aged 6-11 years receiving ChAdOx1 nCoV-19 reported a grade 4 fever of 40·2°C on day 1 following first vaccination, which resolved within 24 h. Pain and tenderness were the most common local solicited adverse events for all the ChAdOx1 nCoV-19 and capsular group B meningococcal groups following both doses. Of the 242 participants with available serostatus data, 14 (6%) were seropositive at baseline. Serostatus data were not available for 20 (8%) of 262 participants. Among seronegative participants who received ChAdOx1 nCoV-19, anti-SARS-CoV-2 IgG and pseudoneutralising antibody titres at day 28 after the second dose were higher in participants aged 12-17 years with a longer interval between doses (geometric means of 73 371 arbitrary units [AU]/mL [95% CI 58 685-91 733] and 299 half-maximal inhibitory concentration [IC50; 95% CI 230-390]) compared with those aged 12-17 years who received their vaccines 28 days apart (43 280 AU/mL [95% CI 35 852-52 246] and 150 IC50 [95% CI 116-194]). Humoral responses were higher in those aged 6-11 years than in those aged 12-17 years receiving their second dose at the same 112-day interval (geometric mean ratios 1·48 [95% CI 1·07-2·07] for anti-SARS-CoV-2 IgG and 2·96 [1·89-4·62] for pseudoneutralising antibody titres). Cellular responses peaked after a first dose of ChAdOx1 nCoV-19 across all age and interval groups and remained above baseline after a second vaccination.
Interpretation: ChAdOx1 nCoV-19 is well tolerated and immunogenic in children aged 6-17 years, inducing concentrations of antibody that are similar to those associated with high efficacy in phase 3 studies in adults. No safety concerns were raised in this trial.
Funding: AstraZeneca and the UK Department of Health and Social Care through the UK National Institute for Health and Care Research.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of interests AJP is Chair of the UK Department of Health and Social Care's Joint Committee on Vaccination and Immunisation but does not participate in policy advice on coronavirus vaccines and was a member of the WHO Strategic Advisory Group of Experts. AJP is a National Institute for Health and Care Research (NIHR) Senior Investigator and is Chief Investigator on clinical trials of Oxford University's COVID-19 vaccine funded by NIHR. TL is named as an inventor on a patent application covering the ChAdOx1 nCoV-19 vaccine and is an occasional consultant to Vaccitech, unrelated to this work. Oxford University has entered into a partnership with AstraZeneca for further development of ChAdOx1 nCoV-19. MDS acts on behalf of the University of Oxford as an investigator on studies funded or sponsored by vaccine manufacturers, including AstraZeneca, GlaxoSmithKline, Pfizer, Novavax, Janssen, Medimmune, and MCM. He receives no personal financial payment for this work. SNF acts on behalf of University Hospital Southampton NHS Foundation Trust as an investigator or provides consultative advice on clinical trials and studies of COVID-19 and other vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, GlaxoSmithKline, Novavax, Seqirus, Sanofi, Medimmune, Merck, and Valneva. He receives no personal financial payment for this work. PTH acts on behalf of St George's University of London as an investigator on clinical trials of COVID-19 vaccines funded or sponsored by vaccine manufacturers, including Janssen, Pfizer, AstraZeneca, Novavax, Moderna, and Valneva. He receives no personal financial payment for this work. All other authors declare no competing interests. AstraZeneca reviewed the final manuscript before submission, but the authors retained editorial control.
Figures
Comment in
-
Increasing children's global access to COVID-19 vaccines.Lancet. 2022 Jun 11;399(10342):2171-2173. doi: 10.1016/S0140-6736(22)00884-4. Lancet. 2022. PMID: 35691308 Free PMC article. No abstract available.
Similar articles
-
Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial.Lancet. 2021 Dec 19;396(10267):1979-1993. doi: 10.1016/S0140-6736(20)32466-1. Epub 2020 Nov 19. Lancet. 2021. PMID: 33220855 Free PMC article. Clinical Trial.
-
Single-dose administration and the influence of the timing of the booster dose on immunogenicity and efficacy of ChAdOx1 nCoV-19 (AZD1222) vaccine: a pooled analysis of four randomised trials.Lancet. 2021 Mar 6;397(10277):881-891. doi: 10.1016/S0140-6736(21)00432-3. Epub 2021 Feb 19. Lancet. 2021. PMID: 33617777 Free PMC article.
-
Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial.Lancet. 2020 Aug 15;396(10249):467-478. doi: 10.1016/S0140-6736(20)31604-4. Epub 2020 Jul 20. Lancet. 2020. PMID: 32702298 Free PMC article. Clinical Trial.
-
Immunity after COVID-19 vaccination in people with higher risk of compromised immune status: a scoping review.Cochrane Database Syst Rev. 2022 Aug 9;8(8):CD015021. doi: 10.1002/14651858.CD015021. Cochrane Database Syst Rev. 2022. PMID: 35943061 Free PMC article.
-
Covid-19 Vaccines Available in India.Comb Chem High Throughput Screen. 2022;25(14):2391-2397. doi: 10.2174/1386207325666220315115953. Comb Chem High Throughput Screen. 2022. PMID: 35293291 Review.
Cited by
-
Adverse events following immunization of COVID-19 vaccine among children aged 6-11 years.Front Public Health. 2022 Oct 25;10:999354. doi: 10.3389/fpubh.2022.999354. eCollection 2022. Front Public Health. 2022. PMID: 36388348 Free PMC article.
-
COVID-19 vaccine update: vaccine effectiveness, SARS-CoV-2 variants, boosters, adverse effects, and immune correlates of protection.J Biomed Sci. 2022 Oct 15;29(1):82. doi: 10.1186/s12929-022-00853-8. J Biomed Sci. 2022. PMID: 36243868 Free PMC article. Review.
-
Humoral and cellular immunity of two-dose inactivated COVID-19 vaccination in Chinese children: A prospective cohort study.J Med Virol. 2023 Jan;95(1):e28380. doi: 10.1002/jmv.28380. J Med Virol. 2023. PMID: 36478357 Free PMC article.
-
Pre-Clinical Development of an Adenovirus Vector Based RSV and Shingles Vaccine Candidate.Vaccines (Basel). 2023 Nov 2;11(11):1679. doi: 10.3390/vaccines11111679. Vaccines (Basel). 2023. PMID: 38006010 Free PMC article.
-
Immunogenicity and Safety of ChAdOx1 nCoV-19 (AZD1222) as a Homologous Fourth-Dose Booster: A Substudy of the Phase 3 COV003 Trial in Brazil.Mayo Clin Proc Innov Qual Outcomes. 2025 Jul 11;9(4):100642. doi: 10.1016/j.mayocpiqo.2025.100642. eCollection 2025 Aug. Mayo Clin Proc Innov Qual Outcomes. 2025. PMID: 40686533 Free PMC article.
References
-
- London School of Hygiene & Tropical Medicine COVID-19 vaccine tracker. 2021. https://vac-lshtm.shinyapps.io/ncov_vaccine_landscape/ - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

