Seroprevalence of SARS-CoV-2 on health professionals via Bayesian estimation: a Brazilian case study before and after vaccines
- PMID: 35691330
- PMCID: PMC9181309
- DOI: 10.1016/j.actatropica.2022.106551
Seroprevalence of SARS-CoV-2 on health professionals via Bayesian estimation: a Brazilian case study before and after vaccines
Abstract
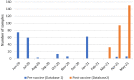
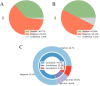
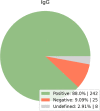
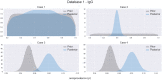
The increasing number of COVID-19 infections brought by the current pandemic has encouraged the scientific community to analyze the seroprevalence in populations to support health policies. In this context, accurate estimations of SARS-CoV-2 antibodies based on antibody tests metrics (e.g., specificity and sensitivity) and the study of population characteristics are essential. Here, we propose a Bayesian analysis using IgA and IgG antibody levels through multiple scenarios regarding data availability from different information sources to estimate the seroprevalence of health professionals in a Northeastern Brazilian city: no data available, data only related to the test performance, data from other regions. The study population comprises 432 subjects with more than 620 collections analyzed via IgA/IgG ELISA tests. We conducted the study in pre- and post-vaccination campaigns started in Brazil. We discuss the importance of aggregating available data from various sources to create informative prior knowledge. Considering prior information from the USA and Europe, the pre-vaccine seroprevalence means are 8.04% and 10.09% for IgG and 7.40% and 9.11% for IgA. For the post-vaccination campaign and considering local informative prior, the median is 84.83% for IgG, which confirms a sharp increase in the seroprevalence after vaccination. Additionally, stratification considering differences in sex, age (younger than 30 years, between 30 and 49 years, and older than 49 years), and presence of comorbidities are provided for all scenarios.
Keywords: Bayesian inference; COVID-19; Databases; Serological Diagnosis; Seroprevalence.
Copyright © 2022. Published by Elsevier B.V.
Conflict of interest statement
None.
Figures
Similar articles
-
Seroprevalence of anti-SARS-CoV-2 IgG antibodies pre- and post-COVID-19 vaccination in staff members of Bandar Abbas Children's Hospital.BMC Infect Dis. 2024 Feb 23;24(1):253. doi: 10.1186/s12879-023-08863-z. BMC Infect Dis. 2024. PMID: 38395759 Free PMC article.
-
Seroprevalence of antibodies against SARS-CoV-2 in the school community in Campo Grande, state of Mato Grosso do Sul, Brazil, October 2021-November 2022.Front Immunol. 2024 Mar 26;15:1354786. doi: 10.3389/fimmu.2024.1354786. eCollection 2024. Front Immunol. 2024. PMID: 38596680 Free PMC article.
-
A Saliva-Based Serological and Behavioral Analysis of SARS-CoV-2 Antibody Prevalence in Howard County, Maryland.Microbiol Spectr. 2023 Aug 17;11(4):e0276522. doi: 10.1128/spectrum.02765-22. Epub 2023 Jun 8. Microbiol Spectr. 2023. PMID: 37289070 Free PMC article.
-
Seroprevalence of SARS-CoV-2 in Brazil: A systematic review and meta-analysis.Clinics (Sao Paulo). 2023 Jun 13;78:100233. doi: 10.1016/j.clinsp.2023.100233. eCollection 2023. Clinics (Sao Paulo). 2023. PMID: 37348256 Free PMC article.
-
SARS-CoV-2 seroprevalence worldwide: a systematic review and meta-analysis.Clin Microbiol Infect. 2021 Mar;27(3):331-340. doi: 10.1016/j.cmi.2020.10.020. Epub 2020 Oct 24. Clin Microbiol Infect. 2021. PMID: 33228974 Free PMC article.
Cited by
-
The effect of COVID vaccination timing on the seroprevalence of IgG antibodies: evidence from the Guayas region of Ecuador.Front Public Health. 2025 Mar 25;13:1537049. doi: 10.3389/fpubh.2025.1537049. eCollection 2025. Front Public Health. 2025. PMID: 40201360 Free PMC article.
References
-
- Alserehi H.A., Alqunaibet A.M., Al-Tawfiq J.A., Alharbi N.K., Alshukairi A.N., Alanazi K.H., Bin Saleh G.M., Alshehri A.M., Almasoud A., Hashem A.M., Alruwaily A.R., Alaswad R.H., Al-Mutlaq H.M., Almudaiheem A.A., Othman F.M., Aldakeel S.A., Abu Ghararah M.R., Jokhdar H.A., Algwizani A.R., Almudarra S.S., Albarrag A.M. Seroprevalence of SARS-CoV-2 (COVID-19) among healthcare workers in Saudi Arabia: comparing case and control hospitals. Diagn. Microbiol. Infect. Dis. 2021;99 doi: 10.1016/j.diagmicrobio.2020.115273. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

