Digital interventions to improve adherence to maintenance medication in asthma
- PMID: 35691614
- PMCID: PMC9188849
- DOI: 10.1002/14651858.CD013030.pub2
Digital interventions to improve adherence to maintenance medication in asthma
Abstract
Background: Asthma is the most common chronic lung condition worldwide, affecting 334 million adults and children globally. Despite the availability of effective treatment, such as inhaled corticosteroids (ICS), adherence to maintenance medication remains suboptimal. Poor ICS adherence leads to increased asthma symptoms, exacerbations, hospitalisations, and healthcare utilisation. Importantly, suboptimal use of asthma medication is a key contributor to asthma deaths. The impact of digital interventions on adherence and asthma outcomes is unknown.
Objectives: To determine the effectiveness of digital interventions for improving adherence to maintenance treatments in asthma.
Search methods: We identified trials from the Cochrane Airways Trials Register, which contains studies identified through multiple electronic searches and handsearches of other sources. We also searched trial registries and reference lists of primary studies. We conducted the most recent searches on 1 June 2020, with no restrictions on language of publication. A further search was run in October 2021, but studies were not fully incorporated.
Selection criteria: We included randomised controlled trials (RCTs) including cluster- and quasi-randomised trials of any duration in any setting, comparing a digital adherence intervention with a non-digital adherence intervention or usual care. We included adults and children with a clinical diagnosis of asthma, receiving maintenance treatment.
Data collection and analysis: We used standard methodological procedures for data collection. We used GRADE to assess quantitative outcomes where data were available.
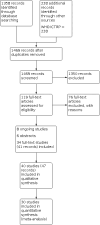
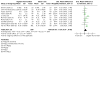
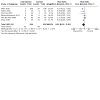
Main results: We included 40 parallel randomised controlled trials (RCTs) involving adults and children with asthma (n = 15,207), of which eight are ongoing studies. Of the included studies, 30 contributed data to at least one meta-analysis. The total number of participants ranged from 18 to 8517 (median 339). Intervention length ranged from two to 104 weeks. Most studies (n = 29) reported adherence to maintenance medication as their primary outcome; other outcomes such as asthma control and quality of life were also commonly reported. Studies had low or unclear risk of selection bias but high risk of performance and detection biases due to inability to blind the participants, personnel, or outcome assessors. A quarter of the studies had high risk of attrition bias and selective outcome reporting. We examined the effect of digital interventions using meta-analysis for the following outcomes: adherence (16 studies); asthma control (16 studies); asthma exacerbations (six studies); unscheduled healthcare utilisation (four studies); lung function (seven studies); and quality of life (10 studies). Pooled results showed that patients receiving digital interventions may have increased adherence (mean difference of 14.66 percentage points, 95% confidence interval (CI) 7.74 to 21.57; low-certainty evidence); this is likely to be clinically significant in those with poor baseline medication adherence. Subgroup analysis by type of intervention was significant (P = 0.001), with better adherence shown with electronic monitoring devices (EMDs) (23 percentage points over control, 95% CI 10.84 to 34.16; seven studies), and with short message services (SMS) (12 percentage points over control, 95% CI 6.22 to 18.03; four studies). No significant subgroup differences were seen for interventions having an in-person component versus fully digital interventions, adherence feedback, one or multiple digital components to the intervention, or participant age. Digital interventions were likely to improve asthma control (standardised mean difference (SMD) 0.31 higher, 95% CI 0.17 to 0.44; moderate-certainty evidence) - a small but likely clinically significant effect. They may reduce asthma exacerbations (risk ratio 0.53, 95% CI 0.32 to 0.91; low-certainty evidence). Digital interventions may result in a slight change in unscheduled healthcare utilisation, although some studies reported no or a worsened effect. School or work absence data could not be included for meta-analysis due to the heterogeneity in reporting and the low number of studies. They may result in little or no difference in lung function (forced expiratory volume in one second (FEV1)): there was an improvement of 3.58% predicted FEV1, 95% CI 1.00% to 6.17%; moderate-certainty evidence); however, this is unlikely to be clinically significant as the FEV1 change is below 12%. Digital interventions likely increase quality of life (SMD 0.26 higher, 95% CI 0.07 to 0.45; moderate-certainty evidence); however, this is a small effect that may not be clinically significant. Acceptability data showed positive attitudes towards digital interventions. There were no data on cost-effectiveness or adverse events. Our confidence in the evidence was reduced by risk of bias and inconsistency.
Authors' conclusions: Overall, digital interventions may result in a large increase in adherence (low-certainty evidence). There is moderate-certainty evidence that digital adherence interventions likely improve asthma control to a degree that is clinically significant, and likely increase quality of life, but there is little or no improvement in lung function. The review found low-certainty evidence that digital interventions may reduce asthma exacerbations. Subgroup analyses show that EMDs may improve adherence by 23% and SMS interventions by 12%, and interventions with an in-person element and adherence feedback may have greater benefits for asthma control and adherence, respectively. Future studies should include percentage adherence as a routine outcome measure to enable comparison between studies and meta-analysis, and use validated questionnaires to assess adherence and outcomes.
Trial registration: ClinicalTrials.gov NCT01529697 NCT04339296 NCT03930381 NCT03907410 NCT03788395 NCT04250779 NCT03951714 NCT03734861.
Copyright © 2022 The Cochrane Collaboration. Published by John Wiley & Sons, Ltd.
Conflict of interest statement
Chan A: Amy Chan has received research grants from A+ charitable trust, Auckland Academic HeaIth Alliance, Chorus, Health Research Council of New Zealand, Maurice Phyllis Paykel Trust, New Zealand Pharmacy and Educational Research Trust, Oakley Mental Health Foundation, Universitas 21, and the University of Auckland. Amy has provided freelance consultancy and received an educational grant from Johnson and Johnson. She is affiliated with the Asthma UK Centre for Applied Research and is supported by Asthma UK (AUK‐AC‐2012‐01 and AUK‐AC‐2018‐01). Amy has received subcontracts from Hong Kong University and from the World Health Organization via University College London to conduct studies unrelated to this review, and provides freelance consultancy to the UCL‐Business spin‐out company Spoonful of Sugar Limited. Amy is also on the board of Asthma NZ and the recipient of the Robert Irwin Foundation fellowship.
De Simoni A: Anna is affiliated with the Asthma UK Centre for Applied Research, and conducted this work with support of the Asthma Research (AUK‐AC‐2012‐01 and AUK‐AC‐2018‐01). Anna has been supported by the National Institute for Health Research. The views expressed are those of the author and not necessarily those of the NHS, NIHR or Department of Health and Social Care.
Wileman V: none known.
Holliday L: Lois is affiliated with the Asthma UK Centre for Applied Research, and conducted this work with the support of the Asthma UK Centre for Applied Research (AUK‐AC‐2012‐01 and AUK‐AC‐2018‐01). Lois has been supported by the National Institute for Health Research. The views expressed are those of the author and not necessarily those of the NHS, NIHR or Department of Health and Social Care.
Newby C: Chris is affiliated with the Asthma UK Centre for Applied Research, and conducted this work with the support of the Asthma UK Centre for Applied Research (AUK‐AC‐2012‐01 and AUK‐AC‐2018‐01). Chris received payment for externally reviewing a PhD Viva for the College of Medicine and Veterinary Medicine.
Chisari C: none known.
Ali S: none known.
Zhu N: none known.
Padakanti P: none known.
Pinprachanan V: none known.
Ting V: none known.
Griffiths C: Chris is supported by the National Institute for Health Research ARC North Thames. The views expressed in this publication are those of the author(s) and not necessarily those of the National Institute for Health and Care Research or the Department of Health and Social Care.
Figures




























Comment in
-
Digitale dimser: svaret på udfordringen med adhærens inden for astmabehandling?Ugeskr Laeger. 2022 Oct 17;184(42):V205122. Ugeskr Laeger. 2022. PMID: 36305264 Danish. No abstract available.
References
References to studies included in this review
Bender 2010 {published data only}
-
- Bender BG, Apter A, Bogen DK, Dickinson P, Fisher L, Wamboldt FS, et al. Test of an interactive voice response intervention to improve adherence to controller medications in adults with asthma. Journal of the American Board of Family Medicine 2010;23(2):159-65. - PubMed
Bender 2015 {published data only}
-
- Bender BG, Cvietusa PJ, Goodrich GK, Lowe R, Nuanes HA, Rand C, et al. Pragmatic trial of health care technologies to improve adherence to pediatric asthma treatment: a randomized clinical trial. JAMA Pediatrics 2015;169(4):317-23. - PubMed
Black 2008 {published data only}
-
- Black PN, Garratt E, Arandjus C, Salmon BT, Sutherland G. An inhaler with ring tones improves compliance with inhaled steroids in childhood asthma. In: American Thoracic Society International Conference. Vol. Poster A46. 2008.
Chan 2015 {published data only}
-
- Chan AHY, Stewart AW, Harrison J, Camargo Jr CA, Black PN, Mitchell EA. The effect of an electronic monitoring device with audiovisual reminder function on adherence to inhaled corticosteroids and school attendance in children with asthma: a randomised controlled trial. Lancet Respiratory Medicine 2015;3(3):210-9. - PubMed
Charles 2007 {published data only}
-
- Charles T, Quinn D, Weatherall M, Aldington S, Beasley R, Holt S. An audiovisual reminder function improves adherence with inhaled corticosteroid therapy in asthma. Journal of Allergy and Clinical Immunology 2007;119(4):811-6. - PubMed
Choi 2017 {published data only}
-
- Choi B, Lee S, Jung J, Suh D. Impact of patient education on medication on health outcomes and adherence in patients with asthma EMT - adult. Allergy: European Journal of Allergy and Clinical Immunology 2017;72:380-1(Abstract 1513).
Clerisme‐Beaty 2011 {published data only}
Cvietusa 2012 {published data only}
-
- Cvietusa Pj, Magid Dj, Goodrich G, Wagner N, Lowe R, Nuanes H, et al. A speech recognition (SR) reminder system improves adherence to ICS among pediatric asthma patients [Abstract]. Journal of Allergy and Clinical Immunology 2012;129(2):AB142 (537).
Davis 2019 {published data only}
-
- Davis SA, Carpenter D, Lee C, Garcia N, Reuland DS, Tudor G, et al. Effect of an asthma question prompt list and video intervention on adolescents' medication adherence 12 months later. Annals of Pharmacotherapy 2019;53(7):683-9. - PubMed
Ebrahimabadi 2019 {published data only}
-
- Ebrahimabadi M, Rezaei K, Moini A, Fournier A, Abedi A. Infographics or video; which one is more effective in asthmatic patients' health? a randomized clinical trial. Journal of Asthma 2019;56(12):1306-13. - PubMed
Foster 2014 {published data only}
-
- Foster JM, Usherwood T, Smith L, Sawyer SM, Xuan W, Rand CS, et al. Inhaler reminders improve adherence with controller treatment in primary care patients with asthma. Journal of Allergy and Clinical Immunology 2014;134(6):1260-8.e3. - PubMed
Jan 2007 {published data only}
-
- Jan RL, Wang JY, Huang MC, Tseng SM, Su HJ, Liu LF. An internet-based interactive telemonitoring system for improving childhood asthma outcomes in Taiwan. Telemedicine Journal and e-Health 2007;13(3):257-68. - PubMed
Johnson 2016a {published data only}
Joseph 2018 {published data only}
-
- Joseph CLM, Mahajan P, Stokes-Buzzelli S, Johnson DA, Duffy E, Williams R, et al. Pilot study of a randomized trial to evaluate a Web-based intervention targeting adolescents presenting to the emergency department with acute asthma. Pilot and Feasibility Studies 2018;4:5. [DOI: 10.1186/s40814-017-0147-6] - DOI - PMC - PubMed
Kenyon 2018 {published data only}
Kim 2016 {published data only}
Kolmodin MacDonell 2016 {published data only}
Kosse 2019 {published data only}
-
- Kosse R, Bouvy M, De Vries T, Koster E. Mobile health intervention increases adherence in adolescents with asthma: a cluster randomised controlled trial in community pharmacies. Journal of Pharmacy Practice 2018;16:1.
-
- Kosse RC, Bouvy ML, Vries TW, Koster ES. Effect of a mHealth intervention on adherence in adolescents with asthma: a randomized controlled trial. Respiratory Medicine 2019;149:45-51. - PubMed
Koufopoulos 2016 {published data only}
Lv 2012 {published data only}
-
- Lv Y, Zhao H, Liang Z, Dong H, Liu L, Zhang D, et al. A mobile phone short message service improves perceived control of asthma: a randomized controlled trial. Telemedicine and E-Health 2012;18(6):420-6. - PubMed
Lv 2019 {published data only}
-
- Lv S, Ye X, Wang Z, Xia W, Qi Y, Wang W, et al. A randomized controlled trial of a mobile application-assisted nurse-led model used to improve treatment outcomes in children with asthma. Journal of Advanced Nursing 2019;75(11):3058-67. - PubMed
Morrison 2016 {published data only}
Morton 2017 {published data only}
-
- Morton R, Everard M, Elphick H. Randomised control trial to investigate whether electronic adherence monitoring with reminder alarms and feedback can improve clinical outcomes in childhood asthma EMT. In: European Respiratory Journal. OA4772 edition. Vol. 46. 2015.
-
- Morton RW, Elphick HE, Rigby AS, Daw WJ, King DA, Smith LJ, et al. STAAR: a randomised controlled trial of electronic adherence monitoring with reminder alarms and feedback to improve clinical outcomes for children with asthma. Thorax 2017;72(4):347-54. - PubMed
Mosnaim 2013 {published data only}
-
- Mosnaim G. Using mobile phones to improve adherence to inhaled steroids (ADEPT4). clinicaltrials.gov/ct2/show/NCT01710059 (first received 18 October 2012).
Pernell 2017 {published data only}
Petrie 2012 {published data only}
-
- Petrie KJ, Perry K, Broadbent E, Weinman J. A text message programme designed to modify patients’ illness and treatment beliefs improves self-reported adherence to asthma preventer medication. British Journal of Health Psychology 2012;17(1):74-84. - PubMed
Pool 2017 {published data only}
Reece 2017 {published data only}
-
- Reece ER, Burnette AF, Lewis-Land C. Pilot study of Asthmawin mobile iphone app in the management of asthma. Journal of Allergy and Clinical Immunology 2017;139(2):AB382.
Rikkers‐Mutsaerts 2012 {published data only}
-
- Rikkers-Mutsaerts E, Winters AE, Bakker MJ, Van Stel HF, Van Der Meer V, De Jongste JC, et al. Internet-based self-management compared with usual care in adolescents with asthma: a randomized controlled trial. Pediatric Pulmonology 2012;47(12):1170-9. - PubMed
Schaffer 2004 {published data only}
-
- Schaffer SD, Tian L. Promoting adherence: effects of theory-based asthma education. Clinical Nursing Research 2004;13(1):69-89. - PubMed
Searing 2012 {published data only}
-
- Searing DA, Bender BG. Short message service (SMS) for asthma management: a pilot study utilizing text messaging to promote asthma self-management. Journal of Allergy and Clinical Immunology 2012;129(2 Suppl):AB142.
Stranbygaard 2010 {published data only}
-
- Strandbygaard U, Backer V. Does daily SMS increase adherence to asthma treatment? A three months follow-up study [Abstract]. In: European Respiratory Society Annual Congress, Vienna, Austria, September 12-16. 2009:E4558.
-
- Strandbygaard U, Thomsen SF, Backer V. A daily SMS reminder increases adherence to asthma treatment: a three-month follow-up study. Respiratory Medicine 2010;104(2):166-71. - PubMed
Sulaiman 2018 {published data only}
-
- Sulaiman I, Greene G, MacHale E, Seheult J, Mokoka M, D’Arcy S, et al. A randomised clinical trial of feedback on inhaler adherence and technique in patients with severe uncontrolled asthma. European Respiratory Journal 2018;51(1):1701126. - PubMed
Van der Meer 2009 {published data only}ISRCTN79864465
-
- Van Der Meer V, Bakker MJ, Van Den Hout WB, Rabe KF, Sterk PJ, Kievit J, et al. Internet-based self-management plus education compared with usual care in asthma a randomized trial. Annals of Internal Medicine 2009;151(2):110-20. - PubMed
Van Sickle 2016 {published data only}
-
- Van Sickle D, Barrett M, Humblet O, Henderson K, Hogg C. Randomized, controlled study of the impact of a mobile health tool on asthma SABA use, control and adherence. European Respiratory Journal 2016;48:PA1018.
Vasbinder 2016 {published data only}
-
- Goossens LMA, Vasbinder EC, Van den Bemt PMLA, Rutten-van Mölken MPMH. Cost-effectiveness of real-time medication monitoring in children with asthma. Value in Health 2014;17(7):PA329. - PubMed
-
- Vasbinder E, Goossens L, Janssens H, Winter B De, Dijk L Van, Vulto A, et al. E-monitoring of asthma therapy to improve compliance in children (E-MATIC). In: European Respiratory Society Annual Congress 2015; Amsterdam, Netherlands. Vol. 46. European Respiratory Society, 2015:OA4771.
-
- Vasbinder EC, Goossens Lucas MA, Rutten-Van Mölken M PMH, De Winter BCM, Van Dijk L, Vulto AG, et al. E-monitoring of asthma therapy to improve compliance in children (e-MATIC): a randomised controlled trial. European Respiratory Journal 2016;48(3):758-67. - PubMed
Vollmer 2011 {published data only}
-
- Vollmer WM, Rand CS, Feldstein AC, Waterbury A, Dubanoski JP, Schneider J, et al. Use of automated phone calls to support inhaled corticosteroids (ICS) adherence [Abstract]. In: American Thoracic Society International Conference 2009 May 15-20; San Diego. A1089 (Poster #518) edition. 2009.
Weinstein 2019 {published data only}
-
- Weinstein AG, Singh A, Laurenceau JP, Skoner DP, Maiolo J, Sharara R, et al. A pilot study of the effect of an educational web application on asthma control and medication adherence. Journal of Allergy and Clinical Immunology 2019;7(5):1497-506. - PubMed
Wiecha 2015 {published data only}
References to studies excluded from this review
Adejumo 2018 {published data only}
-
- Adejumo I, Shaw DE. Electronic monitoring devices as an intervention in asthma: the story so far. Current Respiratory Medicine Reviews 2018;14(1):5-22.
Ahmed 2016 {published data only}
Ainsworth 2019 {published data only}
Anderson 2017 {published data only}
-
- Anderson William C III. Incorporating technology to advance asthma controller adherence. Current Opinion in Allergy and Clinical Immunology 2017;17(2):153-9. - PubMed
Apter 2019 {published data only}
Bender 2018 {published data only}
-
- Bender BG. Technology interventions for nonadherence: new approaches to an old problem. Journal of Allergy and Clinical Immunology 2018;6(3):794-800. - PubMed
Beydon 2017 {published data only}
-
- Beydon N, Delclaux C. Digital action plan for asthma exacerbations (PANAME). Revue des Maladies Respiratoires 2017;34(9):1026-33. - PubMed
Biblowitz 2018 {published data only}
-
- Biblowitz K, Bellam S, Mosnaim G. Improving asthma outcomes in the digital era: a systematic review. Pharmaceutical Medicine 2018;32(3):173-87.
Bonini 2018 {published data only}
-
- Bonini M, Usmani OS. Novel methods for device and adherence monitoring in asthma. Current Opinion in Pulmonary Medicine 2018;24(1):63-9. - PubMed
Boutopoulou 2018 {published data only}
Britto 2017 {published data only}
-
- Britto MT, Rohan JM, Dodds CM, Byczkowski Tl. A randomized trial of user-controlled text messaging to improve asthma outcomes. Clinical Pediatrics 2017;56(14):1336-44. - PubMed
-
- Dodds CM, Britto MT. Learnings from a pragmatic pilot trial of text messaging for high-risk adolescents with asthma. Annals of Allergy, Asthma and Immunology 2018;120(5):546-7. - PubMed
Bruzzese 2021 {published data only}
Chan 2017 {published data only}
-
- Chan AHY, Stewart AW Harrison J, Black PN, Mitchell EA, Foster JM. Electronic adherence monitoring device performance and patient acceptability: a randomized control trial. Expert Review of Medical Devices 2017;14(5):401-11. - PubMed
Chen 2013 {published data only}
-
- Chen SH, Huang JL, Yeh KW, Tsai YF. Interactive support interventions for caregivers of asthmatic children. Journal of Asthma 2013;50(6):649-57. - PubMed
Christakis 2012 {published data only}
Cingi 2015 {published data only}
-
- Cingi C, Yorgancioglu A, Cingi C, Oguzulgen K, Muluk N, Ulusoy, et al. The “physician on call patient engagement trial”(POPET): measuring the impact of a mobile patient engagement application on health outcomes and quality of life in allergic rhinitis and asthma patients. International Forum of Allergy and Rhinology 2015;5:487-97. - PubMed
Claus 2004 {published data only}
-
- Claus R, Michael H, Jan‐Torsten T, Marion S. Internet based patient education evaluation of a new tool for young asthmatics. European Respiratory Journal 2004;24(Suppl 48):383s.
Dermot 2009 {published data only}
-
- Ryan D, Pinnock H, Lee AJ, Tarassenko L, Pagliari Cl, Sheikh A, et al. The CYMPLA trial. Mobile phone-based structured intervention to achieve asthma control in patients with uncontrolled persistent asthma: a pragmatic randomised controlled trial. Primary Care Respiratory Journal 2009;18(4):343. - PMC - PubMed
Dermot 2012 {published data only}
Federman 2018 {published data only}
-
- Federman A, O'Conor R, Mindlis I, Hauser D, Hoy-Rosas J, Lopez R, et al. A comprehensive self-management support program improves asthma control and quality of life among older adults: results of a randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 2018;197:A2714.
Fonseca 2006 {published data only}
-
- Fonseca J, Costa-Pereira A, Delgado L, Fernandes L, Castel-Branco M. Asthma patients are willing to use mobile and web technologies to support self-management. Allergy 2006;61:389-90. - PubMed
Frémont 2018 {published data only}
-
- Frémont A, Abou TR, Wanin S, Lebras M-N, Ollier V, Nathanson S, et al. Cartoons to improve young children's cooperation with inhaled corticosteroids: a preliminary study. Pediatric Pulmonology 2018;53(9):1193-9. - PubMed
Gregoriano 2017 {published data only}
-
- Gregoriano C, Dieterle T, Dürr S, Arnet I, Hersberger KE, Leuppi JD. Impact of an electronic monitoring intervention to improve adherence to inhaled medication in patients with asthma and chronic obstructive pulmonary disease: study protocol for a randomized controlled trial. JMIR Research Protocols 2017;6(10):e204. - PMC - PubMed
Gregoriano 2019 {published data only}
Grossman 2017 {published data only}
-
- Grossman B, Conner S, Mosnaim G, Albers J, Leigh J, Jones S, et al. Application of human augmentics: a persuasive asthma inhaler. Journal of Biomedical Informatics 2017;67:51-8. - PubMed
Gustafson 2012 {published data only}
Halterman 2012 {published data only}
Halterman 2018 {published data only}
Hayter 2019 {published data only}
-
- Haytor V, Ainsworth B. A feasibility study for My Breathing Matters, an asthma self-management website [‘My Breathing Matters’ - A feasibility study of a digital self-management programme designed to improve the quality of life of people with asthma]. trialsearch.who.int/Trial2.aspx?TrialID=ISRCTN15698435 (first received 11 March 2019).
Hew 2019 {published data only}
-
- Hew M, Reddel HK. Integrated adherence monitoring for inhaler medications. JAMA 2019;321(11):1045-6. - PubMed
Hoch 2019 {published data only}
Jeminiwa 2019 {published data only}
-
- Jeminiwa R, Hohmann L, Qian J, Garza K, Hansen R, Fox Brent I. Impact of eHealth on medication adherence among patients with asthma: a systematic review and meta-analysis. Respiratory Medicine 2019;149:59-68. - PubMed
Joseph 2013 {published data only}
Katwa 2018 {published data only}
-
- Katwa U, Rivera E. Asthma management in the era of smart-medicine: devices, gadgets, apps and telemedicine. Indian Journal of Pediatrics 2018;85(9):757-62. - PubMed
Kojima 2005 {published data only}
-
- Kojima N, Takeda Y, Akashi M, Kamiya T, Matsumoto M, Ohya Y, et al. Interactive education during summer camp for children with asthma improved adherence of self-management. Journal of Allergy and Clinical Immunology 2005;115(2):S115.
Koumpagioti 2020 {published data only}
-
- Koumpagioti D, Boutopoulou B, Priftis KN, Douros K. Effectiveness of an educational program for children and their families on asthma control treatment adherence. Journal of Asthma 2020;57(5):567-73. - PubMed
Lathy 2009 {published data only}
-
- Prabhakaran L, Chee J, Kc C, Mun W. The use of text messaging to improve asthma control: A Study of Short Message Service (SMS): PD 10–01 2009–006. Respirology 2009;14:A217.
Lathy 2019 {published data only}
Lau 2015 {published data only}
Licskai 2016 {published data only}
-
- Licskai C, Ferrone M, Taite A, Madeley C, Stevens LA, To T, et al. The evaluation of Breathe-a patient mobile health (mHealth) app for adult asthma. American Journal of Critical Care and Respiratory Medicine 2016;193:A1084.
Lin 2020 {published data only}
Liu 2011 {published data only}
-
- Liu W-Te, Huang C-D, Wang C-H, Lee K-Y, Lin S-M, Kuo H-P. A mobile telephone-based interactive self-care system improves asthma control. European Respiratory Journal 2011;37(2):310-7. - PubMed
Lombard 2019 {published data only}
-
- Lombard L, Walsh J, Plunkett S, MacHale E, Mulvey C, Greene G, et al. INCA (TM) Technology directs the appropriate treatment path for uncontrolled patients with asthma. Irish Journal of Medical Science 2019;188:263.
Makhecha 2019 {published data only}
McPherson 2006 {published data only}
-
- McPherson AC, Glazebrook C, Forster D, James C, Smyth A. A randomized, controlled trial of an interactive educational computer package for children with asthma. Pediatrics 2006;117(4):1046-54. - PubMed
Newhouse 2016 {published data only}
Normansell 2017 {published data only}
Ostojic 2005 {published data only}
-
- Ostojic V, Cvoriscec B, Ostojic SB, Reznikoff D, Stipic-Markovic A, Tudjman Z. Improving asthma control through telemedicine: a study of short-message service. Telemedicine Journal 2005;11(1):28-35. - PubMed
Pearce 2018 {published data only}
-
- Pearce CJ, Fleming L. Adherence to medication in children and adolescents with asthma: methods for monitoring and intervention. Expert Review of Clinical Immunology 2018;14(12):1055-63. - PubMed
Perry 2017 {published data only}
-
- Perry TT, Marshall A, Berlinski A, Rettiganti M, Brown RH, Randle SM, et al. Smartphone-based vs paper-based asthma action plans for adolescents. Annals of Allergy, Asthma and Immunology 2017;118(3):298-303. - PubMed
Poureslami 2017 {published data only}
-
- Poureslami I, Shum J, Gorrin N, Bayat S, Lester RT, Dorscheid D, et al. A randomized controlled trial of a text messaging based intervention versus a written action plan in asthma management: results from a feasibility study. American Journal of Respiratory and Critical Care Medicine 2017;A94:A2637.
Poureslami 2019 {published data only}
-
- Poureslami I, Shum J, Lester RT, Tavakoli H, Dorscheid DR, FitzGerald JM. A pilot randomized controlled trial on the impact of text messaging check-ins and a web-based asthma action plan versus a written action plan on asthma exacerbations. Journal of Asthma 2019;56(8):897-909. - PubMed
Rasmussen 2005 {published data only}
-
- Rasmussen LM, Phanareth K, Nolte H, Backer V. Can internet‐based management improve asthma control? A long term randomised clinical study of 300 asthmatics. European Respiratory Journal 2005;26:400. - PubMed
Real 2019 {published data only}
-
- Francis JR, Beck AF, DeBlasio D, Zackoff M, Henize A, Yingying X, et al. Dose matters: a smartphone application to improve asthma control among patients at an urban pediatric primary care clinic. Games for Health Journal 2019;8(5):357-65. - PubMed
Seid 2012 {published data only}
Stukus 2018 {published data only}
-
- Stukus DR, Farooqui N, Strothman K, Ryan K, Zhao S, Stevens JH, et al. Real-world evaluation of a mobile health application in children with asthma. Annals of Allergy, Asthma and Immunology 2018;120(4):395-400.e1. - PubMed
Sutherland 2017 {published data only}
-
- Sutherland G, Sherlock JP. The potential of connected devices for tackling asthma. Journal of Partnership Opportunities in Drug Delivery 2017:34.
Teufel 2018 {published data only}
Unni 2018 {published data only}
-
- Unni E, Gabriel S, Ariely R. A review of the use and effectiveness of digital health technologies in patients with asthma. Annals of Allergy, Asthma & Immunology 2018;121(6):680-91. - PubMed
Van Gaalen 2013 {published data only}
Voorend‐van Bergan 2015 {published data only}
-
- Voorend-van Bergen S, Vaessen-Verberne AA, Brackel HJ, Landstra AM, den Berg NJ, Hop WC, et al. Monitoring strategies in children with asthma: a randomised controlled trial. Thorax 2015;70(6):543-50. - PubMed
Weinstein 2017 {published data only}
-
- Weinstein A, Gentile D, Singh A, Skoner D, Maiolo J, Sharara R, et al. Preliminary evaluation of an adult asthma adherence management program. American Journal of Respiratory and Critical Care 2017;B38:A7619.
Williams 2010 {published data only}
Yun 2013 {published data only}
-
- Yun T-J, Arriaga RI. A text message a day keeps the pulmonologist away. In: CHI '13: Proceedings of the SIGCHI conference on human factors in computing systems; 2013 27 April -2 May; Paris. 2013.
References to studies awaiting assessment
ACTRN12620001006932 {published data only}
-
- ACTRN12620001006932. The Anti-Inflammatory Reliever Tutorial (AIR-T) sub-study: can an interactive comic tutorial help adult patients with asthma understand and adhere to an anti-inflammatory reliever therapy regimen? An embedded randomised controlled trial. anzctr.org.au/ACTRN12620001006932.aspx (first received 6 August 2020).
Adejumo 2020 {published data only}
-
- Adejumo I, Patel M, McKeever TM, Shaw DE. Feedback on inhaler use does not significantly improve inhaled corticosteroid adherence or clinical outcomes. European Respiratory Journal 2020;56(Suppl 64):4804.
Almonacid 2021 {published data only}
Chen 2020 {published data only}
-
- Chen J, Xu J, Zhao L, Zhang J, Yin Y, Zhang F. The effect of electronic monitoring combined with weekly feedback and reminders on adherence to inhaled corticosteroids in infants and younger children with asthma: a randomized controlled trial. Allergy, Asthma and Clinical Immunology 2020;16(1):68. - PMC - PubMed
CTRI/2021/02/031075 {published data only}
-
- CTRI/2021/02/031075. Effect of education and SMS reminders on medication compliance and quality of life in patients with select multimorbidity (people with 2 or more non communicable diseases)-a randomised controlled trial. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=31236&EncHid=&u... (first received 8 February 2021).
Cvietusa 2020 {published data only}
Ebrahimabadi 2019a {published data only}
-
- Ebrahimabadi M, Rezaei K, Moini A, Fournier A, Abedi A. Infographics or video; which one is more effective in asthmatic patients' health? A randomized clinical trial. Journal of Asthma 2019;56(12):1306-13. - PubMed
EUCTR2019‐003082‐17‐DE {published data only}
-
- EUCTR2019-003082-17-DE. How electronic monitoring and feedback affect use of Easyhaler asthma medication. clinicaltrialsregister.eu/ctr-search/search?query=EUCTR2019-003082-17-DE (first received 29 November 2019).
Gupta 2021 {published data only}
Henderson 2020 {published data only}
Hollenbach 2021 {published data only}
-
- Hollenbach J, Simoneau T, Sun Y, Becene I, Almeida S, Langton C, et al. Design, methods, and baseline characteristics of a pilot, randomized, controlled trial of the effects of an electronic monitoring device on medication adherence in children with asthma. Contemporary Clinical Trials Communications 2021;21:100706. - PMC - PubMed
Lombard 2019a {published data only}
-
- Lombard L, Walsh J, Plunkett S, MacHale E, Mulvey C, Greene G, et al. INCATM technology directs the appropriate treatment path for uncontrolled patients with asthma. Irish Journal of Medical Science 2019;188(Suppl 10):S263.
Moore 2020 {published data only}
Mosnaim 2020 {published data only}
-
- Mosnaim GS, Stempel DA, Gonzalez C, Adams B, BenIsrael-Olive N, Gondalia R, et al. The impact of patient self-monitoring via electronic medication monitor and mobile app plus remote clinician feedback on adherence to inhaled corticosteroids: a randomized controlled trial. Journal of Allergy and Clinical Immunology 2020;9(4):1586-94. - PubMed
NCT04401332 {published data only}
-
- NCT04401332. A randomized controlled trial of apps to home monitor your asthma. clinicaltrials.gov/ct2/show/NCT04401332 (first received 26 May 2020).
NCT04607681 2020 {published data only}
-
- NCT04607681. The effects of web design in educating asthma. clinicaltrials.gov/ct2/show/NCT04607681 (first received 29 October 2020).
NCT04633018 2020 {published data only}
-
- NCT04633018. A patient-centered asthma management communication intervention for rural Latino children. clinicaltrials.gov/ct2/show/NCT04633018 (first received 17 November 2020).
NCT04677959 2020 {published data only}
-
- NCT04677959. A 24-week treatment study to compare standard of care versus the eMDPI DS in patients 13 years or older with asthma. clinicaltrials.gov/ct2/show/NCT04677959 (first received 21 December 2020).
NCT04744272 2021 {published data only}
-
- NCT04744272. Exploring the efficacy of myAsthma in secondary care. clinicaltrials.gov/ct2/show/NCT04744272 (first received 8 February 2021).
NCT04869384 2021 {published data only}
-
- NCT04869384. Effect of electronic monitoring and feedback on adherence to Easyhaler controller medication in patients with asthma. clinicaltrials.gov/ct2/show/NCT04869384 (first received 3 May 2021).
Riley 2021 {published data only}
-
- NCT03769519. AdheRence to Inhaled Corticosteroids in Asthma (ARICA). clinicaltrials.gov/show/NCT03769519 (first received 7 December 2018).
-
- Riley IL, Jackson B, Olsen M, Svetkey L, Que LG, Sanders L, et al. Adherence to inhaled corticosteroids in asthma (ARICA): a randomized controlled pilot study. American Journal of Respiratory and Critical Care Medicine 2021;203(9):A1615.
Sportel 2020 {published data only}
UMIN000042690 {published data only}
-
- UMIN000042690. Using a tracker incorporated into budesonide/formoterol turbuhaler to improve asthma control: a randomized controlled study. upload.umin.ac.jp/cgi-open-bin/ctr_e/ctr_view.cgi?recptno=R000048718 (first received 10 December 2020).
References to ongoing studies
Arain 2020 {published data only}
-
- NCT04339296. Connected healthcare for individuals living at home with chronic conditions [Medication dispensing system to support medication adherence for individuals living at home with chronic conditions: a randomized controlled trial]. clinicaltrials.gov/show/NCT04339296 (first received 20 October2020).
Jariwala 2018 {unpublished data only}
-
- NCT03930381. Adapting and expanding the asthma-educator app [Adapting and expanding the algorithmic software tool to help manage asthma (ASTHMAXcel) for youth with asthma]. clinicaltrials.gov/show/NCT03930381 (first received 29 April 2019).
Kang‐Cheng Su Su 2015 {published data only}
-
- NCT02556073. ICS/LABA combination with integrated dose counter and smartphone app to improve asthma control [The use of fluticasone propionate/salmeterol inhaler with integrated dose counter and smartphone self management to improve airway inflammation and asthma control]. clinicaltrials.gov/show/NCT02556073 (first received 22 September 2015).
Kenyon 2019 {published data only}
-
- NCT03907410. The tailored adherence incentives for childhood asthma medications (TAICAM) trial. clinicaltrials.gov/show/NCT03907410 (first received 9 April 2019).
La Grutta 2020 {published data only}
-
- NCT03788395. The use of an innovative device for therapeutic adherence in pediatric asthma. clinicaltrials.gov/show/NCT03788395 (first received 27 December 2018).
Landon 2019 {published data only}
-
- NCT04250779. Evaluating efficacy of smart device in assisting with inhaler technique and adherence (MOMMIASTHMA1). clinicaltrials.gov/show/NCT04250779 (first received 31 January 2020).
Linnhoff 2019 {published data only}
-
- NCT03951714. Effect of the use of an add-on device connected to a smartphone app on difficult-to-treat asthmatic patient's adherence (ADVICE) [12-wks randomised controlled trial to explore the effect of a smartphone app. Connected to an add-on device system fitted on pMDI inhaler on adherence to medications intake and clinical outcomes in difficult-to-treat asthmatic patients]. clinicaltrials.gov/show/NCT03951714 (first received 15 May 2019).
Simoneau 2018 {published data only}
-
- NCT03734861. The study is enrolling kids from 7 to 16 years old. The BreathSmart Device attaches to the inhaler to measure adherence [A prospective, randomized, controlled study to assess medication adherence in children with asthma managed on BreatheSmart and feedback]. clinicaltrials.gov/show/NCT03734861 (first received 8 November2018).
Additional references
Aaron 2017
-
- Aaron SD, Vandemheen KL, FitzGerald J, Ainslie M, Gupta S, Lemière C, et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA 2017;317(3):269-79. - PubMed
Ali 2014
Alquran 2018
Anderson 2016
Angst 2017
-
- Angst F, Aeschlimann A, Angst J. The minimal clinically important difference raised the significance of outcome effects above the statistical level, with methodological implications for future studies. Journal of Clinical Epidemiology 2017;82:128-36. - PubMed
Asthma Foundation NZ
-
- Asthma Foundation NZ. Asthma and Respiratory Foundation NZ Adolescent and Adult Asthma Guidelines 2020. www.asthmafoundation.org.nz/resources/nz-adolescent-and-adult-asthma-gui... (accessed prior to 14 October 2021).
Babic 2019
Baptist 2016
-
- Baptist AP, Islam N, Joseph CL. Technology-based interventions for asthma - can they help decrease health disparities? Journal of Allergy and Clinical Immunology 2016;4(6):1135-42. - PubMed
Barnes 1993
-
- Barnes PJ. Anti-inflammatory therapy for asthma. Annual Review of Medicine 1993;44(1):229-42. - PubMed
Barnes 2003
-
- Barnes PJ, Adcock IM. How do corticosteroids work in asthma? Annals of Internal Medicine 2003;139(5 Pt 1):359-70. - PubMed
Barnes 2015
-
- Barnes CB, Ulrik CS. Asthma and adherence to inhaled corticosteroids: current status and future perspectives. Respiratory Care 2015;60(3):455-68. - PubMed
Barroso 2018
-
- Barroso AT, Martín EM, Romero LMR, Ruiz FO. Factors affecting lung function: a review of the literature. Archivos de Bronconeumología (English Edition) 2018;54(6):327-32. - PubMed
Bastawrous 2013
Blakey 2018
Britto 2012
-
- Britto MT, Munafo JK, Schoettker PJ, Vockell AL, Wimberg JA, Yi MS. Pilot and feasibility test of adolescent-controlled text messaging reminders. Clinical Pediatrics 2012;51(2):114-21. - PubMed
BTS/SIGN 2016
-
- British Thoracic Society Scottish Intercollegiate Guidelines Network. SIGN158 British guideline on the management of asthma. www.brit-thoracic.org.uk/quality-improvement/guidelines/asthma/ (accessed 7 February 2022).
Bussey‐Smith 2007
-
- Bussey-Smith KL, Rossen RD. A systematic review of randomized control trials evaluating the effectiveness of interactive computerized asthma patient education programs. Annals of Allergy, Asthma and Immunology 2007;98(6):507-16. - PubMed
CDC 2016
-
- Centers for Disease Control and Prevention (CDC). Common asthma triggers. www.cdc.gov/asthma/triggers.html (accessed 14 December 2017).
Chan 2013
-
- Chan AHY, Reddel HK, Apter A, Eakin M, Riekert K, Foster JM. Adherence monitoring and e-health: how clinicians and researchers can use technology to promote inhaler adherence for asthma. Journal of Allergy and Clinical Immunology 2013;1(5):446-54. - PubMed
Clifford 2008
-
- Clifford S, Barber N, Horne R. Understanding different beliefs held by adherers, unintentional nonadherers, and intentional nonadherers: application of the Necessity-Concerns Framework. Journal of Psychosomatic Research 2008;64(1):41-6. - PubMed
Conn 2017
Cooper 2015
-
- Cooper V, Metcalf L, Versnel J, Upton J, Walker S, Horne R. Patient-reported side effects, concerns and adherence to corticosteroid treatment for asthma, and comparison with physician estimates of side-effect prevalence: a UK-wide, cross-sectional study. NPJ Primary Care Respiratory Medicine 2015;25:15026. - PMC - PubMed
D'Arcy 2014
Dayer 2013
Desalu 2021
-
- Desalu O, Ozoh O. Achieving asthma control in low-middle-income countries: why it is important? Journal of the Pan African Thoracic Society 2021;2(2):59-60.
Dima 2015
-
- Dima AL, Hernandez G, Cunillera O, Ferrer M, Bruin M. Asthma inhaler adherence determinants in adults: systematic review of observational data. European Respiratory Journal 2015;45(4):994-1018. - PubMed
Eakin 2012
Ebmeier 2017
-
- Ebmeier S, Thayabaran D, Braithwaite I, Bénamara Clément, Weatherall M, Beasley R. Trends in international asthma mortality: analysis of data from the WHO Mortality Database from 46 countries (1993–2012). Lancet 2017;390(10098):935-45. - PubMed
Engelkes 2015
-
- Engelkes M, Janssens HM, Jongste JC, Sturkenboom MC, Verhamme KM. Medication adherence and the risk of severe asthma exacerbations: a systematic review. European Respiratory Journal 2015;45(2):396-407. - PubMed
Faraone 2008
Farmer 1999
-
- Farmer KC. Methods for measuring and monitoring medication regimen adherence in clinical trials and clinical practice. Clinical Therapeutics 1999;21(6):1074-90. - PubMed
GINA 2017
-
- Global Initiative for Asthma. 2017 GINA Report, Global Strategy for Asthma Management and Prevention. ginasthma.org/wp-content/uploads/2019/04/wmsGINA-2017-main-report-final_... (accessed 14 December 2017).
GINA 2019
-
- Global Initiative for Asthma. 2019 GINA Report, Global Strategy for Asthma Management and Prevention. ginasthma.org/wp-content/uploads/2019/06/GINA-2019-main-report-June-2019... (accessed prior to 15 October 2021).
Global Asthma Report 2014
-
- Global Asthma Network. The Global Asthma Report 2014. www.globalasthmareport.org/ (accessed 14 December 2017).
GRADEpro GDT [Computer program]
-
- GRADEpro GDT. Version accessed before 15 December 2017. Hamilton (ON): McMaster University (developed by Evidence Prime), 2015. Available at gradepro.org.
Higgins 2003
Higgins 2011
-
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Holmes 2014
-
- Holmes EA, Hughes DA, Morrison VL. Predicting adherence to medications using health psychology theories: a systematic review of 20 years of empirical research. Value in Health 2014;17(8):863-76. - PubMed
Hopp 2016
-
- Hopp RJ, Pasha MA. A literature review of the evidence that a 12% improvement in FEV1 is an appropriate cut-off for children. Journal of Asthma 2016;53(4):413-8. [DOI: doi: 10.3109/02770903.2015.1108436] - PubMed
Horne 2005
-
- Horne R, Weinman J, Barber N, Elliott R, Morgan M. Concordance, adherence and compliance in medicine taking. Report for the National Coordinating Centre for NHS Service Delivery and Organization R & D (NCCSDO) (2005). www.netscc.ac.uk/hsdr/files/project/SDO_FR_08-1412-076_V01.pdf (accessed April 2018).
Horne 2019
-
- Horne R, Cooper V, Wileman V, Chan A. Supporting adherence to medicines for long-term conditions: a Perceptions and Practicalities Approach based on an extended Common Sense Model. European Psychologist 2019;24(1):82-96.
Howell 2008
-
- Howell G. Nonadherence to medical therapy in asthma: risk factors, barriers, and strategies for improving. Journal of Asthma 2008;45(9):723-9. - PubMed
Huang 2019
-
- Huang X, Matricardi PM. Allergy and asthma care in the mobile phone era. Clinical Reviews in Allergy and Immunology 2019;56(2):161-73. - PubMed
Johnson 2016b
Jones 2003
-
- Jones C, Santanello NC, Boccuzzi SJ, Wogen J, Strub P, Nelsen LM. Adherence to prescribed treatment for asthma: evidence from pharmacy benefits data. Journal of Asthma 2003;40(1):93-101. [DOI: DOI: 10.1081/JAS-120017212] - PubMed
Joos 2008
-
- Joos S, Miksch A, Szecsenyi J, Wieseler B, Grouven U, Kaiser T, et al. Montelukast as add-on therapy to inhaled corticosteroids in the treatment of mild to moderate asthma: a systematic review. Thorax 2008;63(5):453-62. - PubMed
Kaminsky 2019
-
- Kaminsky, David A. What is a significant bronchodilator response? Annals of the American Thoracic Society 2019;16(12):1495-7. - PubMed
Kannisto 2014
Kardas 2013
Kolmodin 2016
Krebs 2015
Krishna 2003
-
- Krishna S, Francisco BD, Balas EA, König P, Graff GR, Madsen RW. Internet-enabled interactive multimedia asthma education program: a randomized trial. Pediatrics 2003;111(3):503-10. - PubMed
Lasmar 2009
-
- Lasmar L, Camargos P, Champs NS, Fonseca MT, Fontes MJ, Ibiapina C, et al. Adherence rate to inhaled corticosteroids and their impact on asthma control. Allergy 2009;64(5):784-9. - PubMed
Levy 2014
Looijmans‐van den Akker 2016
Maddux 2021
Marcano Belisario 2013
Masoli 2004
-
- Masoli M, Fabian D, Holt S, Beasley R, Global Initiative for Asthma Program. The global burden of asthma: executive summary of the GINA dissemination committee report. Allergy 2004;59(5):469-78. - PubMed
Mathes 2014
Mazumdar 2015
-
- Mazumdar S, Ghosh S, Mukherjee S. Non-adherence to asthma medications: relation to socio-economic status and asthma education. European Respiratory Journal 2015;46:OA4792. [DOI: 10.1183/13993003.congress-2015.OA4792] - DOI
McCambridge 2014
McDonald 2002
-
- McDonald HP, Garg AX, Haynes RB. Interventions to enhance patient adherence to medication prescriptions: scientific review. JAMA 2002;288(22):2868-79. - PubMed
McLean 2016
McQuaid 2012
Menckeberg 2008
-
- Menckeberg TT, Bouvy M L, Bracke M, Kaptein AA, Leufkens HG, Raaijmakers JA, et al. Beliefs about medicines predict refill adherence to inhaled corticosteroids. Journal of Psychosomatic Research 2008;64:47-54. - PubMed
Michie 2017
Moher 2009
Morrison 2014
Mosnaim 2015
Mosnaim 2016
NICE 2021
-
- National Institute for Health and Care Excellence (NICE). Asthma: diagnosis, monitoring and chronic asthma management. NICE guideline [NG80]. www.nice.org.uk/guidance/ng80 (accessed prior to 15 October 2021).
Nieuwlaat 2014
Nolte 2006
-
- Nolte H, Nepper-Christensen S, Backer V. Unawareness and undertreatment of asthma and allergic rhinitis in a general population. Respiratory Medicine 2006;100(2):354-62. - PubMed
Normansell 2018
-
- Normansell R, Chan AHY, Katzer CB, Kew KM, Mes MA, Newby CJ, et al. Health psychology interventions to improve adherence to maintenance therapies in asthma. Cochrane Database of Systematic Reviews 2018, Issue 10. Art. No: CD013147. [DOI: 10.1002/14651858.CD013147] - DOI
Ouzzani 2016
Ponieman 2009
-
- Ponieman D, Wisnivesky JP, Leventhal H, Musumeci-Szabó TJ, Halm EA. Impact of positive and negative beliefs about inhaled corticosteroids on adherence in inner-city asthmatic patients. Annals of Allergy, Asthma and Immunology 2009;103(1):38-42. - PubMed
Ramsey 2020
Reidel 2008
RevMan 2014 [Computer program]
-
- Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riekert 2002
-
- Riekert KA, Rand CS. Electronic monitoring of medication adherence: when is high-tech best? Journal of Clinical Psychology in Medical Settings 2002;9:25-34.
Royal College of Physicians 2014
-
- Royal College of Physicians. Why Asthma Still Kills: The National Review of Asthma Deaths (NRAD) Confidential Enquiry Report (2014). www.rcplondon.ac.uk/projects/national-review-asthma-deaths (accessed 20 April 2018).
Saberi 2011
Sohanpal 2012
Soriano 2017
-
- Soriano JB, Abajobir AA, Abate KH, Abera SF, Agrawal A, Ahmed MB, et al. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respiratory Medicine 2017;5(9):691-706. - PMC - PubMed
Suissa 2000
-
- Suissa S, Ernst P, Benayoun S, Baltzan M, Cai B. Low-dose inhaled corticosteroids and the prevention of death from asthma. New England Journal of Medicine 2000;343:332-6. - PubMed
Sulaiman 2016
-
- Sulaiman I, Seheult J, MacHale E, D'Arcy S, Boland F, McCrory K, et al. Irregular and ineffective: a quantitative observational study of the time and technique of inhaler use. Journal of Allergy and Clinical Immunology 2016;4(5):900-9.e2. - PubMed
Sunstein 2014
-
- Sunstein CR, Thaler RH. Nudge: Improving Decisions About Health, Wealth, and Happiness. Penguin Books, 2014.
Taylor 2018
Thakkar 2016
-
- Thakkar J, Kurup R, Laba T-L, Santo K, Thiagalingam A, Rodgers A, et al. Mobile telephone text messaging for medication adherence in chronic disease: a meta-analysis. JAMA Internal Medicine 2016;176(3):340-9. - PubMed
Tran 2014
-
- Tran N, Coffman JM, Sumino K, Cabana MD. Patient reminder systems and asthma medication adherence: a systematic review. Journal of Asthma 2014;51:536-43. - PubMed
Undela 2021
van Dulmen 2007
van Schayck 2000
Van Steenis 2014
WHO 2003
-
- World Health Organization. Adherence to Long-term Therapies: Evidence for Action. Geneva: World Health Organization, 2003.
WHO 2013
-
- World Health Organization. Asthma fact sheets. who.int/mediacentre/factsheets/fs307/en/ (accessed 14 December 2017).
Williams 2004
-
- Williams LK, Pladevall M, Xi H, Peterson EL, Joseph C, Lafata JE, et al. Relationship between adherence to inhaled corticosteroids and poor outcomes among adults with asthma. Journal of Allergy and Clinical Immunology 2004;114:1288-93. - PubMed

