On the origin and evolution of the asteroid Ryugu: A comprehensive geochemical perspective
- PMID: 35691845
- PMCID: PMC9246647
- DOI: 10.2183/pjab.98.015
On the origin and evolution of the asteroid Ryugu: A comprehensive geochemical perspective
Abstract
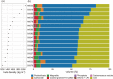
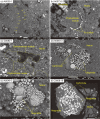
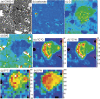
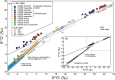
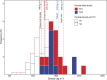
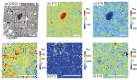
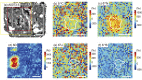
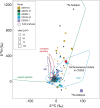
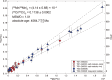
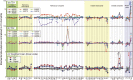
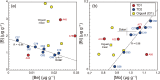
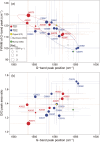
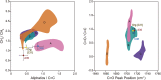
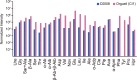
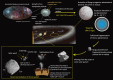
Presented here are the observations and interpretations from a comprehensive analysis of 16 representative particles returned from the C-type asteroid Ryugu by the Hayabusa2 mission. On average Ryugu particles consist of 50% phyllosilicate matrix, 41% porosity and 9% minor phases, including organic matter. The abundances of 70 elements from the particles are in close agreement with those of CI chondrites. Bulk Ryugu particles show higher δ18O, Δ17O, and ε54Cr values than CI chondrites. As such, Ryugu sampled the most primitive and least-thermally processed protosolar nebula reservoirs. Such a finding is consistent with multi-scale H-C-N isotopic compositions that are compatible with an origin for Ryugu organic matter within both the protosolar nebula and the interstellar medium. The analytical data obtained here, suggests that complex soluble organic matter formed during aqueous alteration on the Ryugu progenitor planetesimal (several 10's of km), <2.6 Myr after CAI formation. Subsequently, the Ryugu progenitor planetesimal was fragmented and evolved into the current asteroid Ryugu through sublimation.
Keywords: Hayabusa2; Ryugu; comprehensive analysis; interstellar medium; protosolar nebula; sample return.
Figures










References
-
- Kawaguchi J., Uesugi K., Fujiwara A. (2003) The MUSES-C mission for the sample and return—its technology development status and readiness. Acta Astronaut. 52, 117–123.
-
- Tsuda Y., Saiki T., Terui F., Nakazawa S., Yoshikawa M., Watanabe S., et al. (2020) Hayabusa2 mission status: Landing, roving and cratering on asteroid Ryugu. Acta Astronaut. 171, 42–54.
-
- Kitazato K., Milliken R.E., Iwata T., Abe M., Ohtake M., Matsuura S., et al. (2019) The surface composition of asteroid 162173 Ryugu from Hayabusa2 near-infrared spectroscopy. Science 364, eaav7432. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

