Effectiveness of the third dose of BNT162b2 vaccine on neutralizing Omicron variant in the Japanese population
- PMID: 35691864
- PMCID: PMC9186405
- DOI: 10.1016/j.jiac.2022.05.009
Effectiveness of the third dose of BNT162b2 vaccine on neutralizing Omicron variant in the Japanese population
Abstract
Introduction: The vaccine against SARS-CoV-2 provides humoral immunity to fight COVID-19; however, the acquired immunity gradually declines. Booster vaccination restores reduced humoral immunity; however, its effect on newly emerging variants, such as the Omicron variant, is a concern. As the waves of COVID-19 cases and vaccine programs differ between countries, it is necessary to know the domestic effect of the booster.
Methods: Serum samples were obtained from healthcare workers (20-69 years old) in the Pfizer BNT162b2 vaccine program at the Toyama University Hospital 6 months after the second dose (6mA2D, n = 648) and 2 weeks after the third dose (2wA3D, n = 565). The anti-SARS-CoV-2 antibody level was measured, and neutralization against the wild-type and variants (Delta and Omicron) was evaluated using pseudotyped viruses. Data on booster-related events were collected using questionnaires.
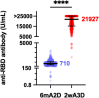
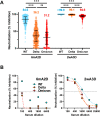
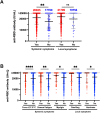
Results: The median anti-SARS-CoV-2 antibody was >30.9-fold elevated after the booster (6mA2D, 710.0 U/mL [interquartile range (IQR): 443.0-1068.0 U/mL]; 2wA3D, 21927 U/mL [IQR: 15321.0->25000.0 U/mL]). Median neutralizing activity using 100-fold sera against wild-type-, Delta-, and Omicron-derived variants was elevated from 84.6%, 36.2%, and 31.2% at 6mA2D to >99.9%, 99.1%, and 94.6% at 2wA3D, respectively. The anti-SARS-CoV-2 antibody levels were significantly elevated in individuals with fever ≥37.5 °C, general fatigue, and myalgia, local swelling, and local hardness.
Conclusion: The booster effect, especially against the Omicron variant, was observed in the Japanese population. These findings contribute to the precise understanding of the efficacy and side effects of the booster and the promotion of vaccine campaigns.
Keywords: Anti-receptor binding domain antibody; BNT162b2; Booster; Neutralizing antibody; Omicron.
Copyright © 2022 Japanese Society of Chemotherapy and The Japanese Association for Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Figures
References
-
- Munro A.P.S., Janani L., Cornelius V., Aley P.K., Babbage G., Baxter D., et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of Chadox1 nCOV-19 or BNT162b2 in the UK (cov-boost): a blinded, multicentre, randomised, controlled, phase 2 trial. Lancet. 2021;398:2258–2276. - PMC - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

