Safety and immunogenicity of anti-SARS CoV-2 vaccine SOBERANA 02 in homologous or heterologous scheme: Open label phase I and phase IIa clinical trials
- PMID: 35691871
- PMCID: PMC9167831
- DOI: 10.1016/j.vaccine.2022.05.082
Safety and immunogenicity of anti-SARS CoV-2 vaccine SOBERANA 02 in homologous or heterologous scheme: Open label phase I and phase IIa clinical trials
Abstract
Background: SOBERANA 02 is a COVID-19 vaccine based on SARS-CoV-2 recombinant RBD conjugated to tetanus toxoid (TT). SOBERANA Plus antigen is dimeric-RBD. Here we report safety and immunogenicity from phase I and IIa clinical trials using two-doses of SOBERANA 02 and three-doses (homologous) or heterologous (with SOBERANA Plus) protocols.
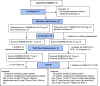
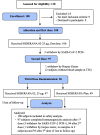
Method: We performed an open-label, sequential and adaptive phase I to evaluate safety and explore the immunogenicity of SOBERANA 02 in two formulations (15 or 25 μg RBD-conjugated to 20 μg of TT) in 40 subjects, 19-59-years-old. Phase IIa was open-label including 100 volunteers 19-80-years, receiving two doses of SOBERANA 02-25 μg. In both trials, half of volunteers were selected to receive a third dose of the corresponding SOBERANA 02 and half received a heterologous dose of SOBERANA Plus. Primary outcome was safety. The secondary outcome was immunogenicity evaluated by anti-RBD IgG ELISA, molecular neutralization of RBD:hACE2 interaction, live-virus-neutralization and specific T-cells response.
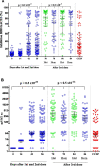
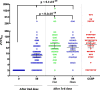
Results: The most frequent adverse event (AE) was local pain, other AEs had frequencies ≤ 5%. No serious related-AEs were reported. Phase IIa confirmed the safety in 60 to 80-years-old subjects. In phase-I SOBERANA 02-25 µg elicited higher immune response than SOBERANA 02-15 µg and progressed to phase IIa. Phase IIa results confirmed the immunogenicity of SOBERANA 02-25 µg even in 60-80-years. Two doses of SOBERANA02-25 µg elicited an immune response similar to that of the Cuban Convalescent Serum Panel and it was higher after the homologous and heterologous third doses. The heterologous scheme showed a higher immunological response. Anti-RBD IgG neutralized the delta variant in molecular assay, with a 2.5-fold reduction compared to D614G neutralization.
Conclusions: SOBERANA 02 was safe and immunogenic in persons aged 19-80 years, eliciting neutralizing antibodies and specific T-cell response. Highest immune responses were obtained in the heterologous three doses protocol.
Trial registry: https://rpcec.sld.cu/trials/RPCEC00000340, https://rpcec.sld.cu/trials/RPCEC00000347.
Keywords: COVID-19; Conjugate vaccine; Heterologous immunization scheme; SARS-CoV-2; Subunit vaccine.
Copyright © 2022 Elsevier Ltd. All rights reserved.
Conflict of interest statement
Declaration of Competing Interest The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: [The Finlay Vaccine Institute, the Centre of Molecular Immunology and the University of Havana have filed patent applications related to the vaccine SOBERANA 02. The authors declare the following competing financial interest(s): L.R.N, B.S.R, R.P.N, S.F.C, Y.C.R, D.S.M, U.R.G, T.B.A, E.O.M, D.G.R, Y.V.B., D.G.R, V.V.B are co-inventors on provisional SARS-CoV-2 vaccine patents (Cu 2020-69). The rest of the authors declare no competing interests. No authors received an honorarium for this paper.]
Figures



References
-
- WHO Coronavirus (COVID-19) Dashboard, https://covid19.who.int/ (Consulted October 20, 2021).
-
- Guidance for Industry: Development and Licensure of Vaccines to Prevent COVID-19, USFDA, June 2020. https://www.fda.gov/regulatory-information/search-fda-guidance-documents... (Consulted October 20, 2021).
-
- Voysey M., Clemens S.A.C., Madhi S.A., Weckx L.Y., Folegatti P.M., Aley P.K., et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

