Changes in software as a medical device based on artificial intelligence technologies
- PMID: 35691995
- PMCID: PMC9188918
- DOI: 10.1007/s11548-022-02669-1
Changes in software as a medical device based on artificial intelligence technologies
Abstract
Purpose: to develop a procedure for registering changes, notifying users about changes made, unifying software as a medical device based on artificial intelligence technologies (SaMD-AI) changes, as well as requirements for testing and inspections-quality control before and after making changes.
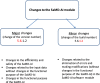
Methods: The main types of changes, divided into two groups-major and minor. Major changes imply a subsequent change of a SaMD-AI version to improve efficiency and safety, to change the functionality, and to ensure the processing of new data types. Minor changes imply those that SaMD-AI developers can make due to errors in the program code. Three types of SaMD-AI testings are proposed to use: functional testing, calibration testing or control, and technical testing.
Results: The presented approaches for validation SaMD-AI changes were introduced. The unified requirements for the request for changes and forms of their submission made this procedure understandable for SaMD-AI developers, and also adjusted the workload for the Experiment experts who checked all the changes made to SaMD-AI.
Conclusion: This article discusses the need to control changes in the module of SaMD-AI, as innovative products influencing medical decision making. It justifies the need to control a module operation of SaMD-AI after making changes. To streamline and optimize the necessary and sufficient control procedures, a systematization of possible changes in SaMD-AI and testing methods was carried out.
Keywords: Artificial intelligence; Changes; Medical software based on artificial intelligence technologies; Modifications; Software as a medical device; Validation.
© 2022. CARS.
Conflict of interest statement
The authors declare they have no financial interests. Morozov S.P. was an unpaid president European Society of Medical Imaging Informatics (till 2018). Morozov S.P. is an unpaid chairman of SC 01 “Artificial Intelligence in Healthcare”.
Figures






References
-
- Ranschaert E, Morozov S, Algra P, editors. Artificial intelligence in medical imaging. Cham: Springer; 2019.
-
- Gusev A, Dobridnyuk S. Artificial intelligence in medicine and healthcare. Inform Soc. 2017;4–5:78–93.
-
- The World Health Organization guidance. Ethics and governance of artificial intelligence for health. https://www.who.int/publications/i/item/9789240029200
-
- Sharova D, Zinchenko V, Akhmad E, Mokienko O, Vladzymyrskyy A, Morozov S. On the issue of ethical aspects of the artificial intelligence systems implementation in healthcare. Dig Diagnost. 2021;2(3):356–368. doi: 10.17816/DD77446. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

