Surface Anchoring and Active Sites of [Mo3S13]2- Clusters as Co-Catalysts for Photocatalytic Hydrogen Evolution
- PMID: 35692252
- PMCID: PMC9171716
- DOI: 10.1021/acscatal.2c00972
Surface Anchoring and Active Sites of [Mo3S13]2- Clusters as Co-Catalysts for Photocatalytic Hydrogen Evolution
Abstract
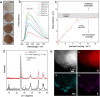
Achieving light-driven splitting of water with high efficiency remains a challenging task on the way to solar fuel exploration. In this work, to combine the advantages of heterogeneous and homogeneous photosystems, we covalently anchor noble-metal- and carbon-free thiomolybdate [Mo3S13]2- clusters onto photoactive metal oxide supports to act as molecular co-catalysts for photocatalytic water splitting. We demonstrate that strong and surface-limited binding of the [Mo3S13]2- to the oxide surfaces takes place. The attachment involves the loss of the majority of the terminal S2 2- groups, upon which Mo-O-Ti bonds with the hydroxylated TiO2 surface are established. The heterogenized [Mo3S13]2- clusters are active and stable co-catalysts for the light-driven hydrogen evolution reaction (HER) with performance close to the level of the benchmark Pt. Optimal HER rates are achieved for 2 wt % cluster loadings, which we relate to the accessibility of the TiO2 surface required for efficient hole scavenging. We further elucidate the active HER sites by applying thermal post-treatments in air and N2. Our data demonstrate the importance of the trinuclear core of the [Mo3S13]2- cluster and suggest bridging S2 2- and vacant coordination sites at the Mo centers as likely HER active sites. This work provides a prime example for the successful heterogenization of an inorganic molecular cluster as a co-catalyst for light-driven HER and gives the incentive to explore other thio(oxo)metalates.
© 2022 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
-
- Bičáková O.; Straka P. Production of Hydrogen from Renewable Resources and Its Effectiveness. Int. J. Hydrogen Energy 2012, 37, 11563–11578. 10.1016/j.ijhydene.2012.05.047. - DOI
-
- Chen S.; Takata T.; Domen K. Particulate Photocatalysts for Overall Water Splitting. Nat. Rev. Mater. 2017, 2, 1–17. 10.1038/natrevmats.2017.50. - DOI
-
- Conway B. E.; Tilak B. V. Interfacial Processes Involving Electrocatalytic Evolution and Oxidation of H2, and the Role of Chemisorbed H. Electrochim. Acta 2002, 47, 3571–3594. 10.1016/S0013-4686(02)00329-8. - DOI
LinkOut - more resources
Full Text Sources
