A Longitudinal Magnetoencephalographic Study of the Effects of Deep Brain Stimulation on Neuronal Dynamics in Severe Anorexia Nervosa
- PMID: 35692383
- PMCID: PMC9178415
- DOI: 10.3389/fnbeh.2022.841843
A Longitudinal Magnetoencephalographic Study of the Effects of Deep Brain Stimulation on Neuronal Dynamics in Severe Anorexia Nervosa
Abstract
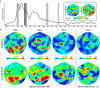
Anorexia Nervosa (AN) is a debilitating psychiatric disorder characterized by the relentless pursuit of thinness, leading to severe emaciation. Magnetoencephalography (MEG)was used to record the neuronal response in seven patients with treatment-resistant AN while completing a disorder-relevant food wanting task. The patients underwent a 15-month protocol, where MEG scans were conducted pre-operatively, post-operatively prior to deep brain stimulation (DBS) switch on, twice during a blind on/off month and at protocol end. Electrodes were implanted bilaterally into the nucleus accumbens with stimulation at the anterior limb of the internal capsule using rechargeable implantable pulse generators. Three patients met criteria as responders at 12 months of stimulation, showing reductions of eating disorder psychopathology of over 35%. An increase in alpha power, as well as evoked power at latencies typically associated with visual processing, working memory, and contextual integration was observed in ON compared to OFF sessions across all seven patients. Moreover, an increase in evoked power at P600-like latencies as well as an increase in γ-band phase-locking over anterior-to-posterior regions were observed for high- compared to low-calorie food image only in ON sessions. These findings indicate that DBS modulates neuronal process in regions far outside the stimulation target site and at latencies possibly reflecting task specific processing, thereby providing further evidence that deep brain stimulation can play a role in the treatment of otherwise intractable psychiatric disorders.
Keywords: N400 & P600; alpha power; anorexia nervosa; deep brain stimulation; magnetoencephalography; phase-locking; treatment.
Copyright © 2022 Braeutigam, Scaife, Aziz and Park.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

