SARS-CoV-2 antibody response after BNT162b2 mRNA vaccine in healthcare workers: Nine-month of follow-up
- PMID: 35692461
- PMCID: PMC9170276
- DOI: 10.1016/j.jvacx.2022.100175
SARS-CoV-2 antibody response after BNT162b2 mRNA vaccine in healthcare workers: Nine-month of follow-up
Abstract
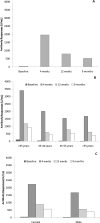
We collected sequential serum samples (0, 4, 12 weeks, 9 months) for the determination of S-RDB IgG levels from 103 vaccinated healthy subjects (age 45 ± 13 years; 60 women), in order to evaluate neutralizing antibody response against SARS-CoV-2 in healthy healthcare workers (HCWs) after the administration of two doses of BNT162b2 SARS-CoV-2 mRNA vaccine. Every subject received two doses of mRNA vaccine BNT162b2 (Pfizer-BioNTech), 21 days apart (January-February 2021). Furthermore, antibody titer of 14 subjects who were hospitalized for symptomatic COVID-19 was evaluated. Antibody response was (median, interquartile range) 35 U/mL (10-104) at baseline, 1960 (1241-3221) at 4 weeks, 791 (388-1179) at 12 weeks and 524 (273-931) at 6 months. Antibody response was inversely correlated with age at all timepoints (p < 0.001) while gender and Body Mass Index had no significant effect. At multivariate analysis, post-baseline values were significantly higher than baseline (p < 0.001) with a reduction at 12 weeks and 9 months (p < 0.001). Antibody response of hospitalized subjects who did not receive vaccination, symptomatic for COVID 19 infection, was 103 (25-557) U/mL, significantly higher than baseline (p = 0.007) of study population but lower than all post-baseline determinations (p < 0.001). Younger subjects showed a stronger response and a lower decrease of antibody titers compared to the classes of older subjects. SARS-CoV2 infection was excluded by performing 1017 nasopharyngeal RT-PCR swabs on the study cohort. The second dose of mRNA vaccine resulted in an antibody response effective in preventing infection in a population of healthcare professionals. The antibody level was stable through week 12, showing a reduction in the following six months.
Keywords: Antibody responses; COVID-19; Vaccine.
© 2022 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Fedele G, Stefanelli P, Bella A, Fiore S, Pancheri S, Benedetti E, Fabiani C, Leone P, Vacca P, Schiavoni I, Neri A, Carannante A, Simmaco M, Santino I, Zuccali MG, Bizzarri G, Magnoni R, Benetollo PP, Brusaferro S, Rezza G, Ferro A. Anti-SARS-CoV-2 antibodies persistence after natural infection: a repeated serosurvey in Northern Italy. Ann Ist Super Sanita. 2021 Oct-Dec;57(4):265-271. doi: 10.4415/ANN_21_04_01. PMID: 35076416. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

