Co-administration of chloroquine and coenzyme Q10 improved treatment outcome during experimental cerebral malaria
- PMID: 35692470
- PMCID: PMC9177935
- DOI: 10.1007/s12639-022-01468-4
Co-administration of chloroquine and coenzyme Q10 improved treatment outcome during experimental cerebral malaria
Abstract
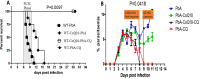
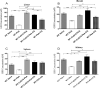
Development of cerebral malaria (CM) is driven by parasitemia levels, harmful inflammatory response, oxidative stress and consequent breach of the blood brain barrier. Use of adjunct therapy that utilizes an antioxidant and anti-inflammatory agent alongside chloroquine (CQ), may improve treatment outcome and shorten recovery from post-infection sequelae. Though withdrawn in some countries, CQ is still in use for prophylaxis and treatment of malaria in many countries. Current study investigated whether oral co-administration of 50 mg/kg CQ and 200 mg/kg of coenzyme Q10 (CoQ10) would improve treatment outcome against experimental cerebral malaria (ECM) and assuage the deleterious effects of oxidative stress and inflammation upon infection by Plasmodium berghei ANKA (PbA) in a C57BL/6 J mouse model. Treatment with CQ + CoQ10 resulted in an improved parasite elimination; clearing the parasite one day early, when compared to mice on CQ alone. Remarkably, treatment with CQ and CoQ10 separately or in combination, assuaged PbA induced elevation of serum levels of TNF-α and IFN-γ an indication of protection from ECM progression. Furthermore, CQ and CoQ10-administration, blocked parasite-driven elevation of aspartate transaminase (AST), alanine transaminase (ALT) and bilirubin. In the presence of CQ and CoQ10, severe PbA-induced systemic induction of oxidative stress and resultant GSH depletion was reduced in the brain, liver, spleen, and kidney. Overall, these findings demonstrate that administration of CQ and CoQ10 ameliorates harmful parasite-driven oxidative stress and inflammation, while slowing the progression to full blown ECM and may improve treatment outcome in CM.
Keywords: Chloroquine; Coenzyme Q10; Experimental cerebral malaria; Inflammation; Oxidative stress.
© Indian Society for Parasitology 2022.
Conflict of interest statement
Conflict of interestThere was no any form of conflict of interest reported by authors arising from this study.
Figures



References
LinkOut - more resources
Full Text Sources
