Multimodal MRI-Based Radiomics-Clinical Model for Preoperatively Differentiating Concurrent Endometrial Carcinoma From Atypical Endometrial Hyperplasia
- PMID: 35692806
- PMCID: PMC9186045
- DOI: 10.3389/fonc.2022.887546
Multimodal MRI-Based Radiomics-Clinical Model for Preoperatively Differentiating Concurrent Endometrial Carcinoma From Atypical Endometrial Hyperplasia
Abstract
Objectives: To develop and validate a radiomics model based on multimodal MRI combining clinical information for preoperative distinguishing concurrent endometrial carcinoma (CEC) from atypical endometrial hyperplasia (AEH).
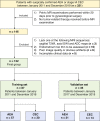
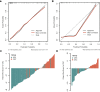
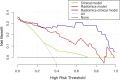
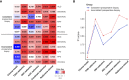
Materials and methods: A total of 122 patients (78 AEH and 44 CEC) who underwent preoperative MRI were enrolled in this retrospective study. Radiomics features were extracted based on T2-weighted imaging (T2WI), diffusion-weighted imaging (DWI), and apparent diffusion coefficient (ADC) maps. After feature reduction by minimum redundancy maximum relevance and least absolute shrinkage and selection operator algorithm, single-modal and multimodal radiomics signatures, clinical model, and radiomics-clinical model were constructed using logistic regression. Receiver operating characteristic (ROC) analysis, calibration curves, and decision curve analysis were used to assess the models.
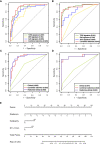
Results: The combined radiomics signature of T2WI, DWI, and ADC maps showed better discrimination ability than either alone. The radiomics-clinical model consisting of multimodal radiomics features, endometrial thickness >11mm, and nulliparity status achieved the highest area under the ROC curve (AUC) of 0.932 (95% confidential interval [CI]: 0.880-0.984), bootstrap corrected AUC of 0.922 in the training set, and AUC of 0.942 (95% CI: 0.852-1.000) in the validation set. Subgroup analysis further revealed that this model performed well for patients with preoperative endometrial biopsy consistent and inconsistent with postoperative pathologic data (consistent group, F1-score = 0.865; inconsistent group, F1-score = 0.900).
Conclusions: The radiomics model, which incorporates multimodal MRI and clinical information, might be used to preoperatively differentiate CEC from AEH, especially for patients with under- or over-estimated preoperative endometrial biopsy.
Keywords: endometrial hyperplasia; endometrial neoplasms; magnetic resonance imaging; radiomics; texture analysis.
Copyright © 2022 Zhang, Zhang, Wang, Song, Yu, Xie, Chen and Ouyang.
Conflict of interest statement
Author LX was employed by GE Healthcare. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures

References
-
- Baak JP, Mutter GL, Robboy S, van Diest PJ, Uyterlinde AM, Orbo A, et al. The Molecular Genetics and Morphometry-Based Endometrial Intraepithelial Neoplasia Classification System Predicts Disease Progression in Endometrial Hyperplasia More Accurately Than the 1994 World Health Organization Classification System. Cancer (2005) 103(11):2304–12. doi: 10.1002/cncr.21058 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources

