Correlation of Phenotype-Genotype and Protein Structure in RYR1-Related Myopathy
- PMID: 35693006
- PMCID: PMC9178086
- DOI: 10.3389/fneur.2022.870285
Correlation of Phenotype-Genotype and Protein Structure in RYR1-Related Myopathy
Abstract
Introduction: Next generation sequencing results in an explosive identification of rare variants of RYR1, making the correlation between phenotype and genotype complicated. We analyzed the data of 33 patients with RYR1-related myopathy, attempting to elucidate correlations between phenotype, genotype, and protein structure of RyR1.
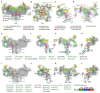
Methods: Clinical, histopathologic, and genetic data were evaluated, and variants were mapped to the cryo-EM RyR1 structure. The three-dimensional structure of the variant on RyR1 was analyzed.
Results: The clinical spectrum was highly variable regardless of the mode of inheritance. Recessive variations were associated with more severe feeding problems and respiratory insufficiency in infancy (p < 0.05). Forty pathogenic and likely pathogenic variations were identified, and 14 of them were novel. Missense was the most common variation type regardless of inheritance mode. Arginine (15/45) was the most frequently involved residue. All but one dominant variation clustered in Pore forming and pVSD domains, while recessive variations enriched in Bsol (7/25) and SPRYs (6/25) domains. Analysis of the spatial structure of variants showed that dominant variants may impact RyR1 mainly by breaking down hydrogen or electrovalent bonds (10/21); recessive variants located in different domains may impact the function of RyR1 through different pathways. Variants located in RyR1 coupling sites (PY1&2 and the outermost of Bsol) may cause the most severe clinical manifestation.
Conclusion: Clinical diversity of RYR1-related myopathy was impacted by the inheritance mode, variation type, and variant location. Dominant and recessive variants have different sensitive domains impacting the function of RyR1 through different pathways.
Keywords: RYR1-related myopathy; cohort study; genotype; phenotype; protein structure.
Copyright © 2022 Chang, Wei, Wei, Liu, Qin, Yan, Ma, Wang and Xiong.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
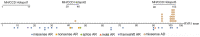

References
LinkOut - more resources
Full Text Sources

