Microglia in Alzheimer's Disease: A Favorable Cellular Target to Ameliorate Alzheimer's Pathogenesis
- PMID: 35693110
- PMCID: PMC9184163
- DOI: 10.1155/2022/6052932
Microglia in Alzheimer's Disease: A Favorable Cellular Target to Ameliorate Alzheimer's Pathogenesis
Abstract
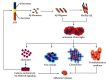
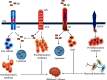
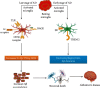
Microglial cells serve as molecular sensors of the brain that play a role in physiological and pathological conditions. Under normal physiology, microglia are primarily responsible for regulating central nervous system homeostasis through the phagocytic clearance of redundant protein aggregates, apoptotic cells, damaged neurons, and synapses. Furthermore, microglial cells can promote and mitigate amyloid β phagocytosis and tau phosphorylation. Dysregulation of the microglial programming alters cellular morphology, molecular signaling, and secretory inflammatory molecules that contribute to various neurodegenerative disorders especially Alzheimer's disease (AD). Furthermore, microglia are considered primary sources of inflammatory molecules and can induce or regulate a broad spectrum of cellular responses. Interestingly, in AD, microglia play a double-edged role in disease progression; for instance, the detrimental microglial effects increase in AD while microglial beneficiary mechanisms are jeopardized. Depending on the disease stages, microglial cells are expressed differently, which may open new avenues for AD therapy. However, the disease-related role of microglial cells and their receptors in the AD brain remain unclear. Therefore, this review represents the role of microglial cells and their involvement in AD pathogenesis.
Copyright © 2022 Dewan Md. Sumsuzzman et al.
Conflict of interest statement
The authors proclaim no conflict of interest.
Figures
Similar articles
-
Metabolic regulation of microglial phagocytosis: Implications for Alzheimer's disease therapeutics.Transl Neurodegener. 2023 Oct 31;12(1):48. doi: 10.1186/s40035-023-00382-w. Transl Neurodegener. 2023. PMID: 37908010 Free PMC article. Review.
-
Fibrillar Aβ triggers microglial proteome alterations and dysfunction in Alzheimer mouse models.Elife. 2020 Jun 8;9:e54083. doi: 10.7554/eLife.54083. Elife. 2020. PMID: 32510331 Free PMC article.
-
Microglial Lyzl4 Facilitates β-Amyloid Clearance in Alzheimer's Disease.Adv Sci (Weinh). 2025 Jan;12(2):e2412184. doi: 10.1002/advs.202412184. Epub 2024 Nov 18. Adv Sci (Weinh). 2025. PMID: 39555667 Free PMC article.
-
Targeting Microglia in Alzheimer's Disease: Pathogenesis and Potential Therapeutic Strategies.Biomolecules. 2024 Jul 11;14(7):833. doi: 10.3390/biom14070833. Biomolecules. 2024. PMID: 39062547 Free PMC article. Review.
-
Environmental Enrichment Potently Prevents Microglia-Mediated Neuroinflammation by Human Amyloid β-Protein Oligomers.J Neurosci. 2016 Aug 31;36(35):9041-56. doi: 10.1523/JNEUROSCI.1023-16.2016. J Neurosci. 2016. PMID: 27581448 Free PMC article.
Cited by
-
Ginkgolide attenuates memory impairment and neuroinflammation by suppressing the NLRP3/caspase-1 pathway in Alzheimer's disease.Aging (Albany NY). 2023 Oct 3;15(19):10237-10252. doi: 10.18632/aging.205072. Epub 2023 Oct 3. Aging (Albany NY). 2023. PMID: 37793010 Free PMC article.
-
Association Between Porphyromonas gingivalis and Alzheimer's Disease: A Comprehensive Review.Infect Drug Resist. 2025 Apr 25;18:2119-2136. doi: 10.2147/IDR.S491628. eCollection 2025. Infect Drug Resist. 2025. PMID: 40308631 Free PMC article. Review.
-
Recent advances in Alzheimer's disease: Mechanisms, clinical trials and new drug development strategies.Signal Transduct Target Ther. 2024 Aug 23;9(1):211. doi: 10.1038/s41392-024-01911-3. Signal Transduct Target Ther. 2024. PMID: 39174535 Free PMC article. Review.
-
Microglial Modulation in Alzheimer's Disease: Central Players in Neuroinflammation and Pathogenesis.Curr Alzheimer Res. 2025;22(1):56-82. doi: 10.2174/0115672050364292250113063513. Curr Alzheimer Res. 2025. PMID: 39976100 Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

