Comparison of laboratory parameters in mild vs. severe cases and died vs. survived patients with COVID-19: systematic review and meta-analysis
- PMID: 35693606
- PMCID: PMC9186220
- DOI: 10.21037/jtd-22-345
Comparison of laboratory parameters in mild vs. severe cases and died vs. survived patients with COVID-19: systematic review and meta-analysis
Abstract
Background: This study aimed to summarize the available data on the association between the severity of (COVID-19) and routine blood indicators, inflammatory, biochemical parameters and coagulation parameter.
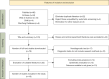
Methods: A literature search was conducted of PubMed, EMBASE, and Web of Sciences, CNKI, WanFang database providing relevant data. Random-effects meta-analysis was used to pool effect sizes.
Results: In patients with severe symptoms, interleukin-6, [IL-6; standardized mean difference (SMD) =1.15, 95% confidence interval (95% CI): 1.01, 1.29, P<0.001, n=1,121], interleukin-10 (IL-10; SMD =0.92, 95% CI: 0.75, 1.08, P<0.001, n=782), interleukin-4 (IL-4; SMD =0.2, 95% CI: 0.01, 0.39, P=0.04, n=500), procalcitonin (PCT; SMD =1.16, 95% CI: 0.99, 1.33, P<0.001, n=734), C-reactive protein (CRP; SMD =1.42, 95% CI: 1.27, 1.57, P<0.001, n=1,286), serum amyloid A (SAA; SMD =2.82, 95% CI: 2.53, 3.11, P<0.001, n=502) neutrophil count (SMD =0.63, 95% CI: 0.44, 0.82, P<0.001, n=558), alanine aminotransferase (ALT; SMD =2.72, 95% CI: 2.43, 3.02, P<0.001, n=538), aspartate aminotransferase (AST; SMD =2.75, 95% CI: 2.37, 3.12, P<0.001, n=313), lactate dehydrogenase (LDH; SMD =4.01, 95% CI: 3.79, 4.24, P<0.001, n=1,055), creatine kinase (CK; SMD =2.62, 95% CI: 2.2, 3.03, P<0.001, n=230;), CK-MB isoenzyme (CK-MB; SMD =3.07, 95% CI: 2.81, 3.34, P<0.001, n=600, activated partial thromboplastin time (APTT; SMD =0.63, 95% CI: 0.39, 0.87, P<0.001, n=351), and prothrombin time (P-T; SMD =1.83, 95% CI: 1.55, 2.11, P<0.001, n=351) were significantly higher than in patients with mild symptoms. On the contrary, lymphocyte count (SMD =-1.04, 95% CI: -1.21, -0.86, P<0.001, n=805) platelets (SMD =-1.47, 95% CI: -1.7, -1.24, P<0.001, n=653), monocyte count (SMD =-0.56, 95% CI: -0.8, -0.32, P<0.001, n=403), and albumin (SMD =-2.95, 95% CI: -3.21, -2.7, P<0.001, n=637) was significantly lower in patients with severe symptoms than in patients with mild symptoms. IL-6 (SMD =2.62, 95% CI: 2.15, 3.09, P<0.001, n=185), PCT (SMD =0.2, 95% CI: 0.16, 0.23, P<0.001, n=156), creatinine (SMD =2.29, 95% CI: 1.87, 2.7, P<0.001, n=213), and neutrophil counts (SMD =2.77, 95% CI: 2.38, 3.16, P<0.001, n=260) in patients with COVID-19 in the death group were significantly higher than that in patients in the survival group, while the lymphocyte count was significantly lower.
Conclusions: In summary, current evidence show that those laboratory indicators are associated with the severity of COVID-19 and thus could be used as prognostic risk stratification of patients with COVID-19.
Keywords: Coronavirus disease 2019 (COVID-19); laboratory biomarker; meta-analysis; prognosis.
2022 Journal of Thoracic Disease. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://jtd.amegroups.com/article/view/10.21037/jtd-22-345/coif). The authors have no conflicts of interest to declare.
Figures
References
-
- Yang P, Ding Y, Xu Z, et al. Epidemiological and clinical features of COVID-19 patients with and without pneumonia in Beijing, China. bioRxiv 2020. doi: . 10.1101/2020.02.28.20028068 - DOI
-
- Taghiloo S, Soltanshahi M, Aliyali M, et al. Cytokine profiling in Iranian patients with COVID-19; association with clinical severity. Iran J Immunol 2021;18:54-64. - PubMed
-
- Metlay JP, Waterer GW, Long AC, et al. Diagnosis and Treatment of Adults with Community-acquired Pneumonia. An Official Clinical Practice Guideline of the American Thoracic Society and Infectious Diseases Society of America. Am J Respir Crit Care Med 2019;200:e45-e67. 10.1164/rccm.201908-1581ST - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous