Recent advances in connexin gap junction biology
- PMID: 35693635
- PMCID: PMC9171797
- DOI: 10.12703/r/11-14
Recent advances in connexin gap junction biology
Abstract
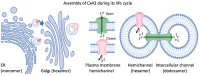
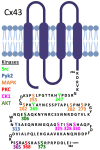
Connexins are assembled into dodecamer intercellular channels, a collection of which is termed a gap junction, and their canonical function allowing direct exchange of ions and metabolites has been unequivocally established. When initially assembled into undocked cell surface connexin hemichannels, healthy cells may also engage in cell signaling via a regulated small-molecule release. Recent advances in the field have led to an expanded view of the functional roles of intercellular channels and hemichannels in both physiology and pathology. As more of the 21-member human connexin family is intensely interrogated, mounting evidence points to the biological uniqueness of each member, and no longer can we confidently refer to all connexins engaging in the same cellular processes. Innovations in high-resolution cryo-electron microscopy have revealed important insights into the structure of functionally important domains of both hemichannels and channels. These and other studies have established a foundation of knowledge that should allow inhibitory smart drug design for situations where enhanced intercellular or hemichannel activity is at the root of a connexin-linked disease. Assessment of the connexin interactome, which varies widely for each connexin subtype, continues to provide regulatory insights into the assembly and function of connexins that exhibit a short half-life. As the most intensely studied, Cx43 is found in about 50% of all human cell types and is extensively regulated by multiple inhibitory and enhancing phosphorylation events that have direct implications on tissue function and outcomes of disease, including cancer. Here, we briefly discuss these advances and give our thoughts on where the field is headed.
Keywords: channel; connexin; gap junctions; hemichannel.
Copyright: © 2022 Lampe PD et al.
Conflict of interest statement
PDL notes that he is an unpaid member of the Scientific Advisory Board of FirstString Research LLC but states that this service has not influenced this presentation. DWL declares that he has no competing interests.No competing interests were disclosed.No competing interests were disclosed.No competing interests were disclosed.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
