Synthesis and characterization of some novel polythiophene derivatives containing pyrazoline
- PMID: 35693727
- PMCID: PMC9186369
- DOI: 10.1080/15685551.2022.2086413
Synthesis and characterization of some novel polythiophene derivatives containing pyrazoline
Abstract
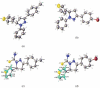
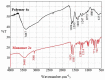
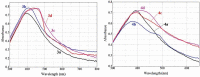
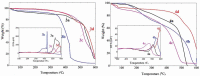
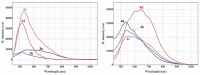
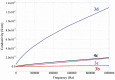
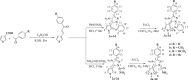
Eight polythiophene derivatives containing pyrazoline side groups were synthesized by a chemical oxidative coupling polymerization using FeCl3. The crystal structures of four monomers were determined which confirm the almost perpendicular orientation of the thiophene and pyrazoline rings, while the other substituents are more coplanar. Analyses of IR, 1H-NMR, Raman and UV-Vis spectra demonstrated that the suggested polymerization was successful to generate the synthesized polythiophenes with the expected structures. The morphology of the synthesized polythiophenes was studied by SEM. The different substituents attached to the 1- and 3-positions of the pyrazoline side chain led to differences in optical properties, electrical conductivity, and thermal stability of the synthesized polythiophenes. By adding a pyrazoline side chain to polythiophenes, some polymers achieve good solubility, electrical conductivity of about 1.3 × 10-6 S/cm, high fluorescence intensity (above 40,000 a.u.) at 505-550 nm and thermal stability up to 590°C in the air.
Keywords: Polythiophene derivatives; chemical polymerization; crystal structure; pyrazoline heterocycle.
© 2022 The Author(s). Published by Informa UK Limited, trading as Taylor & Francis Group.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
-
- Javadi A, Najjar Z, Bahadori S, et al. High refractive index and low-birefringence polyamides containing thiazole and naphthalene units. RSC Adv. 2015;5(111):91670–91682.
-
- Fukuzaki N, Higashihara T, Ando S, et al. Synthesis and characterization of highly refractive polyimides derived from thiophene-containing aromatic diamines and aromatic dianhydrides. Macromolecules. 2010;43(4):1836–1843.
-
- Javadi A, Shockravi A, Rafieimanesh A, et al. Synthesis and structure–property relationships of novel thiazole‐containing poly (amide imide) s with high refractive indices and low birefringences. Poly Int. 2015;64(4):486–495.
-
- Liu JG, Nakamura Y, Terraza CA, et al. Highly refractive polyimides derived from 2, 8‐Bis (p‐aminophenylenesulfanyl) dibenzothiophene and aromatic dianhydrides. Macromol Chem Phys. 2008;209(2):195–203.
-
- Javadi A, Shockravi A, Koohgard M, et al. Nitro-substituted polyamides: a new class of transparent and highly refractive materials. Eur Polym J. 2015;66:328–341.
LinkOut - more resources
Full Text Sources
