A Dissenters' View on AppleSnail Immunobiology
- PMID: 35693764
- PMCID: PMC9178244
- DOI: 10.3389/fimmu.2022.879122
A Dissenters' View on AppleSnail Immunobiology
Abstract
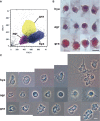
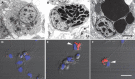
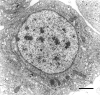
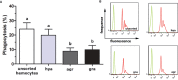
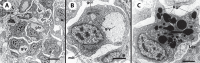
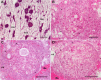
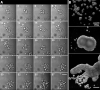
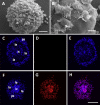
We stand as dissenters against the acceptance of scientific knowledge that has not been built on empirical data. With this in mind, this review synthesizes selected aspects of the immunobiology of gastropods and of apple snails (Ampullariidae) in particular, from morphological to molecular and "omics" studies. Our trip went through more than two centuries of history and was guided by an evo-devo hypothesis: that the gastropod immune system originally developed in the mesenchymal connective tissue of the reno-pericardial complex, and that in that tissue some cells differentiated into hematopoietically committed progenitor cells that integrate constitutive hemocyte aggregations in the reno-pericardial territory, whether concentrated in the pericardium or the kidney in a species-specific manner. However, some of them may be freed from those aggregations, circulate in the blood, and form distant contingent aggregations anywhere in the body, but always in response to intruders (i.e., pathogens or any other immune challenge). After that, we reviewed the incipient immunology of the Ampullariidae by critically revising the findings in Pomacea canaliculata and Marisa cornuarietis, the only ampullariid species that have been studied in this respect, and we attempted to identify the effectors and the processes in which they are involved. Particularly for P. canaliculata, which is by far the most studied species, we ask which hemocytes are involved, in which tissues or organs are integrated, and what cellular reactions to intruders this species has in common with other animals. Furthermore, we wondered what humoral factors could also integrate its internal defense system. Among the cellular defenses, we give an outstanding position to the generation of hemocyte nodules, which seems to be an important process for these snails, serving the isolation and elimination of intruders. Finally, we discuss hematopoiesis in apple snails. There have been contrasting views about some of these aspects, but we envision a hematopoietic system centered in the constitutive hemocyte islets in the ampullariid kidney.
Keywords: Biomphalaria; Pomacea; hematopoiesis; hemocyanin; hemocyte; nodulation; reno-pericardial complex; rhogocyte.
Copyright © 2022 Rodriguez, Vega and Castro-Vazquez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


Similar articles
-
Pathogenesis of an experimental mycobacteriosis in an apple snail.Front Immunol. 2023 Oct 9;14:1253099. doi: 10.3389/fimmu.2023.1253099. eCollection 2023. Front Immunol. 2023. PMID: 37876924 Free PMC article.
-
Both quiescent and proliferating cells circulate in the blood of the invasive apple snail Pomacea canaliculata.Fish Shellfish Immunol. 2020 Dec;107(Pt A):95-103. doi: 10.1016/j.fsi.2020.09.026. Epub 2020 Sep 20. Fish Shellfish Immunol. 2020. PMID: 32966893
-
A prokineticin-like protein responds to immune challenges in the gastropod pest Pomacea canaliculata.Dev Comp Immunol. 2017 Jul;72:37-43. doi: 10.1016/j.dci.2017.02.001. Epub 2017 Feb 3. Dev Comp Immunol. 2017. PMID: 28163091
-
Old species and new concepts in the taxonomy of Pomacea (Gastropoda: Ampullariidae).Biocell. 2002 Apr;26(1):71-81. Biocell. 2002. PMID: 12058383 Review.
-
Gastropod immunobiology.Adv Exp Med Biol. 2010;708:17-43. doi: 10.1007/978-1-4419-8059-5_2. Adv Exp Med Biol. 2010. PMID: 21528691 Review.
Cited by
-
Short-Term Estivation and Hibernation Induce Changes in the Blood and Circulating Hemocytes of the Apple Snail Pomacea canaliculata.Metabolites. 2023 Feb 16;13(2):289. doi: 10.3390/metabo13020289. Metabolites. 2023. PMID: 36837908 Free PMC article.
-
Clodronate Liposome-Mediated Phagocytic Hemocyte Depletion Affects the Regeneration of the Cephalic Tentacle of the Invasive Snail, Pomacea canaliculata.Biology (Basel). 2023 Jul 12;12(7):992. doi: 10.3390/biology12070992. Biology (Basel). 2023. PMID: 37508422 Free PMC article.
-
Pathogenesis of an experimental mycobacteriosis in an apple snail.Front Immunol. 2023 Oct 9;14:1253099. doi: 10.3389/fimmu.2023.1253099. eCollection 2023. Front Immunol. 2023. PMID: 37876924 Free PMC article.
-
SlugAtlas, a histological and 3D online resource of the land slugs Deroceras laeve and Ambigolimax valentianus.PLoS One. 2024 Oct 22;19(10):e0312407. doi: 10.1371/journal.pone.0312407. eCollection 2024. PLoS One. 2024. PMID: 39436899 Free PMC article.
References
-
- Barsanti G. Lamarck and the Birth of Biology 1740–1810. In: Poggi S, Bossi M, editors. Romanticism in Science: Science in Europe, 1790–1840. Dordrecht: Springer Netherlands; (1994). p. 47–74.
-
- Lamarck J-BPAdMd . Histoire Naturelle des Animaux Sans Vertèbres. Paris: au Jardin du Roi: J.B. Baillière; (1822).
-
- Bouchet P, Rocroi J-P, Hausdorf B, Kaim A, Kano Y, Nützel A, et al. . Revised Classification, Nomenclator and Typification of Gastropod and Monoplacophoran Families. Malacologia (2017) 61(1–2):1–526. doi: 10.4002/040.061.0201 - DOI
-
- Pan C-T. Studies on the Biological Control of Schistosome-Bearing Snails: A Preliminary Report on Pathogenic Microorganisms Found in Australorbis glabratus . J Parasitol (1956) 42(4, Sect. 2):33.
-
- Pan C-T. The General Histology and Topographic Microanatomy of Australorbis glabratus . Bull Mus Comp Zool Harv Coll (1958) 119(3):237–99.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

