METTL3-Mediated N6-Methyladenosine Modification of Trim59 mRNA Protects Against Sepsis-Induced Acute Respiratory Distress Syndrome
- PMID: 35693774
- PMCID: PMC9174697
- DOI: 10.3389/fimmu.2022.897487
METTL3-Mediated N6-Methyladenosine Modification of Trim59 mRNA Protects Against Sepsis-Induced Acute Respiratory Distress Syndrome
Abstract
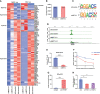
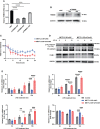
N6-methyladenosine (m6A) RNA modification is a fundamental determinant of mRNA metabolism in eukaryotic cells and is involved in numerous physiological and pathological processes. However, the specific role of m6A modification in sepsis-induced acute respiratory distress syndrome(ARDS) remains unknown. Here, we show that the levels of m6A RNA were significantly decreased in septic lungs and that METTL3 was the main regulator involved in the absence of m6A RNA modification. Pulmonary endothelial barrier damage is a critical process in the pathogenesis of acute lung injury during sepsis. METTL3 regulated endothelial barrier dysfunction and inflammatory responses in sepsis-induced ARDS in vivo and in vitro. Furthermore, we identified tripartite motif-containing (Trim)59 as a key m6A effector and Trim59 deficiency exacerbated lung injury. Mechanistically, METTL3 inhibited endothelial injury in sepsis-induced ARDS through Trim59-associated NF-κB inactivation. Our findings revealed novel insights into epitranscriptional mechanisms in sepsis-induced ARDS via m6A modifications, which has important application value in the diagnosis, prognosis, and molecular-targeted therapy of sepsis-associated lung injury.
Keywords: METTL3; acute lung injury; endothelial barrier; epigenetic regulation; m6A; sepsis.
Copyright © 2022 Chen, Wu, Zhu, Chen, Xu, Tang, Jiao and Yu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

