Bifidobacterium longum Ameliorates Ovariectomy-Induced Bone Loss via Enhancing Anti-Osteoclastogenic and Immunomodulatory Potential of Regulatory B Cells (Bregs)
- PMID: 35693779
- PMCID: PMC9174515
- DOI: 10.3389/fimmu.2022.875788
Bifidobacterium longum Ameliorates Ovariectomy-Induced Bone Loss via Enhancing Anti-Osteoclastogenic and Immunomodulatory Potential of Regulatory B Cells (Bregs)
Abstract
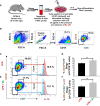
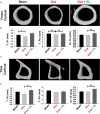
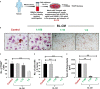
Discoveries in the last few years have emphasized the existence of an enormous breadth of communication between osteo-immune systems. These discoveries fuel novel approaches for the treatment of several bone pathologies including osteoporosis. Bifidobacterium longum (BL) is a preferred probiotic of choice due to its varied immunomodulatory potential in alleviating various inflammatory diseases. Here, we evaluate the effect of BL in an ovariectomy (ovx)-induced post-menopausal osteoporotic mouse model. Our in vitro findings reveal that BL suppresses the differentiation and functional activity of RANKL-induced osteoclastogenesis in both mouse bone marrow cells and human PBMCs. Strikingly, BL-induced Bregs were found to be significantly more efficient in suppressing osteoclastogenesis and modulating Treg-Th17 cell balance with respect to control Bregs in vitro. Our in vivo µCT and bone mechanical strength data further confirm that BL supplementation significantly enhanced bone mass and bone strength, along with improving the bone microarchitecture in ovx mice. Remarkably, alterations in frequencies of CD19+CD1dhiCD5+IL-10+ Bregs, CD4+Foxp3+IL-10+ Tregs, and CD4+Rorγt+IL-17+ Th17 cells in distinct lymphoid organs along with serum-cytokine data (enhanced anti-osteoclastogenic cytokines IFN-γ and IL-10 and reduced osteoclastogenic-cytokines IL-6, IL-17, and TNF-α) strongly support the immunomodulatory potential of BL. Altogether, our findings establish a novel osteo-protective and immunomodulatory potential of BL in augmenting bone health under osteoporotic conditions.
Keywords: Bregs; Th17; Tregs; immunoporosis; osteoporosis; probiotics.
Copyright © 2022 Sapra, Shokeen, Porwal, Saini, Bhardwaj, Mathew, Mishra, Chattopadhyay, Dar, Verma and Srivastava.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures









References
-
- Sapra L, Azam Z, Rani L, Saini C, Bhardwaj A, Shokeen N, et al. “Immunoporosis”: Immunology of Osteoporosis. Proc Natl Acad Sci India Sect B Biol Sci (2021) 91:511–19. doi: 10.1007/s40011-021-01238-x - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

