A SARS-CoV-2 Spike Receptor Binding Motif Peptide Induces Anti-Spike Antibodies in Mice andIs Recognized by COVID-19 Patients
- PMID: 35693806
- PMCID: PMC9178084
- DOI: 10.3389/fimmu.2022.879946
A SARS-CoV-2 Spike Receptor Binding Motif Peptide Induces Anti-Spike Antibodies in Mice andIs Recognized by COVID-19 Patients
Expression of concern in
-
Expression of concern on: A SARS-CoV-2 spike receptor binding motif peptide induces anti-spike antibodies in mice andIs recognized by COVID-19 patients.Front Immunol. 2023 Sep 15;14:1292353. doi: 10.3389/fimmu.2023.1292353. eCollection 2023. Front Immunol. 2023. PMID: 37786613 Free PMC article. No abstract available.
Abstract
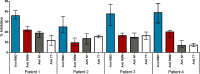
The currently devastating pandemic of severe acute respiratory syndrome known as coronavirus disease 2019 or COVID-19 is caused by the coronavirus SARS-CoV-2. Both the virus and the disease have been extensively studied worldwide. A trimeric spike (S) protein expressed on the virus outer bilayer leaflet has been identified as a ligand that allows the virus to penetrate human host cells and cause infection. Its receptor-binding domain (RBD) interacts with the angiotensin-converting enzyme 2 (ACE2), the host-cell viral receptor, and is, therefore, the subject of intense research for the development of virus control means, particularly vaccines. In this work, we search for smaller fragments of the S protein able to elicit virus-neutralizing antibodies, suitable for production by peptide synthesis technology. Based on the analysis of available data, we selected a 72 aa long receptor binding motif (RBM436-507) of RBD. We used ELISA to study the antibody response to each of the three antigens (S protein, its RBD domain and the RBM436-507 synthetic peptide) in humans exposed to the infection and in immunized mice. The seroreactivity analysis showed that anti-RBM antibodies are produced in COVID-19 patients and immunized mice and may exert neutralizing function, although with a frequency lower than anti-S and -RBD. These results provide a basis for further studies towards the development of vaccines or treatments focused on specific regions of the S virus protein, which can benefit from the absence of folding problems, conformational constraints and other advantages of the peptide synthesis production.
Keywords: COVID-19; SARS-CoV-2; immunized animals; neutralizing Abs; receptor binding motif; spike (S) protein.
Copyright © 2022 Pratesi, Errante, Pacini, Peña-Moreno, Quiceno, Carotenuto, Balam, Konaté, Diakité, Arévalo-Herrera, Kajava, Rovero, Corradin, Migliorini, Papini and Herrera.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
-
- WHO . Weekly Epidemiological Update on COVID-19 (2022). Available at: https://www.who.int/publications/m/item/weekly-epidemiological-update-on....
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

