Efficacy of single-dose HPV vaccination among young African women
- PMID: 35693874
- PMCID: PMC9172784
- DOI: 10.1056/EVIDoa2100056
Efficacy of single-dose HPV vaccination among young African women
Abstract
BACKGROUND: Single-dose human papillomavirus (HPV) vaccination, if efficacious, would be tremendously advantageous, simplifying implementation and decreasing costs. METHODS: We performed a randomized, multicenter, double-blind, controlled trial of single-dose nonavalent (HPV 16/18/31/33/45/52/58/6/11 infection) or bivalent (HPV 16/18 infection) HPV vaccination compared with meningococcal vaccination among Kenyan women 15 to 20 years of age. Enrollment and 6-monthly cervical swabs and a month 3 vaginal swab were tested for HPV deoxyribonucleic acid (DNA). Enrollment sera were tested for HPV antibodies. The modified intent-to-treat (mITT) cohort comprised participants who had an HPV antibody-negative result at enrollment and an HPV DNA-negative result at enrollment and month 3. The primary outcome was incident persistent vaccine-type HPV infection by month 18. RESULTS: Between December 2018 and June 2021, 2275 women were randomly assigned and followed. A total of 758 participants received the nonavalent HPV vaccine, 760 received the bivalent HPV vaccine, and 757 received the meningococcal vaccine; retention was 98%. Thirty-eight incident persistent infections were detected in the HPV 16/18 mITT cohort: one each among participants assigned to the bivalent and nonavalent groups and 36 among those assigned to the meningococcal group. Nonavalent vaccine efficacy (VE) was 97.5% (95% confidence interval [CI], 81.7 to 99.7%; P≤0.0001), and bivalent VE was 97.5% (95% CI, 81.6 to 99.7%; P≤0.0001). Thirty-three incident persistent infections were detected in the HPV 16/18/31/33/45/52/58 mITT cohort: four in the nonavalent group and 29 in the meningococcal group. Nonavalent VE for HPV 16/18/31/33/45/52/58 was 88.9% (95% CI, 68.5 to 96.1; P<0.0001). The rate of serious adverse events was 4.5% to 5.2% by group. CONCLUSIONS: Over the 18-month timeframe we studied, single-dose bivalent and nonavalent HPV vaccines were each highly effective in preventing incident persistent oncogenic HPV infection, similar to multidose regimens. (Funded by the National Institutes of Health, the Bill and Melinda Gates Foundation, and the University of Washington; ClinicalTrials.gov number, NCT03675256.)
Copyright: © 2022 Author(s), Massachusetts Medical Society. All rights reserved.
Figures
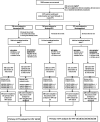
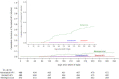
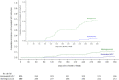
References
-
- Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2021. - PubMed
-
- A Global Strategy for elimination of cervical cancer. 2020. (Accessed 09/20/2020, 2020, at https://www.who.int/activities/a-global-strategy-for-elimination-of-cerv....)
-
- Australia set to eliminate cervical cancer by 2035. 2018. (Accessed April 29, 2019, at https://www.cancer.org.au/news/media-releases/australia-set-to-eliminate....)
-
- Joura EA, Giuliano AR, Iversen OE, et al. A 9-valent HPV vaccine against infection and intraepithelial neoplasia in women. The New England journal of medicine 2015;372:711–23. - PubMed
-
- Koutsky LA, Ault KA, Wheeler CM, et al. A controlled trial of a human papillomavirus type 16 vaccine. The New England journal of medicine 2002;347:1645–51. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
