Standards for practical intravenous rapid drug desensitization & delabeling: A WAO committee statement
- PMID: 35694005
- PMCID: PMC9163606
- DOI: 10.1016/j.waojou.2022.100640
Standards for practical intravenous rapid drug desensitization & delabeling: A WAO committee statement
Abstract
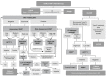
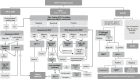
Drug hypersensitivity reactions (DHRs) to intravenous drugs can be severe and might leave patients and doctors in a difficult position where an essential treatment or intervention has to be suspended. Even if virtually any intravenous medication can potentially trigger a life-threatening DHR, chemotherapeutics, biologics, and antibiotics are amongst the intravenous drugs most frequently involved in these reactions. Admittedly, suspending such treatments may negatively impact the survival outcomes or the quality of life of affected patients. Delabeling pathways and rapid drug desensitization (RDD) can help reactive patients stay on first-choice therapies instead of turning to less efficacious, less cost-effective, or more toxic alternatives. However, these are high-complexity and high-risk techniques, which usually need expert teams and allergy-specific techniques (skin testing, in vitro testing, drug provocation testing) to ensure safety, an accurate diagnosis, and personalized management. Unfortunately, there are significant inequalities within and among countries in access to allergy departments with the necessary expertise and resources to offer these techniques and tackle these DHRs optimally. The main objective of this consensus document is to create a great benefit for patients worldwide by aiding allergists to expand the scope of their practice and support them with evidence, data, and experience from leading groups from around the globe. This statement of the Drug Hypersensitivity Committee of the World Allergy Organization (WAO) aims to be a comprehensive practical guide on the technical aspects of implementing acute-onset intravenous hypersensitivity delabeling and RDD for a wide range of drugs. Thus, the manuscript does not only focus on clinical pathways. Instead, it also provides guidance on topics usually left unaddressed, namely, internal validation, continuous quality improvement, creating a healthy multidisciplinary environment, and redesigning care (including a specific supplemental section on a real-life example of how to design a dedicated space that can combine basic and complex diagnostic and therapeutic techniques in allergy).
Keywords: Antibiotic desensitization; Antibiotics; Betalactams; Biological agents; Chemotherapy; Delabeling; Drug allergy; Drug challenge; Drug desensitization; Drug provocation test; Penicillins; Personalized medicine; Precision medicine; Risk stratification; Skin test.
© 2022 The Authors.
Conflict of interest statement
The authors report no competing interests with regards to this document.
Figures


References
-
- Madrigal-Burgaleta R., Bernal-Rubio L., Berges-Gimeno M.P., Carpio-Escalona L.V., Gehlhaar P., Alvarez-Cuesta E. A large single-hospital experience using drug provocation testing and rapid drug desensitization in hypersensitivity to antineoplastic and biological agents. J Allergy Clin Immunol Pract. 2019;7(2):618–632. doi: 10.1016/j.jaip.2018.07.031. - DOI - PubMed
-
- Madrigal-Burgaleta R., Berges-Gimeno M.P., Angel-Pereira D., et al. Hypersensitivity and desensitization to antineoplastic agents: outcomes of 189 procedures with a new short protocol and novel diagnostic tools assessment. Allergy – Eur J Allergy Clin Immunol. 2013;68(7) doi: 10.1111/all.12105. - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources

