Survival of newly formed particles in haze conditions
- PMID: 35694134
- PMCID: PMC9119030
- DOI: 10.1039/d2ea00007e
Survival of newly formed particles in haze conditions
Abstract
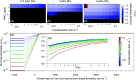
Intense new particle formation events are regularly observed under highly polluted conditions, despite the high loss rates of nucleated clusters. Higher than expected cluster survival probability implies either ineffective scavenging by pre-existing particles or missing growth mechanisms. Here we present experiments performed in the CLOUD chamber at CERN showing particle formation from a mixture of anthropogenic vapours, under condensation sinks typical of haze conditions, up to 0.1 s-1. We find that new particle formation rates substantially decrease at higher concentrations of pre-existing particles, demonstrating experimentally for the first time that molecular clusters are efficiently scavenged by larger sized particles. Additionally, we demonstrate that in the presence of supersaturated gas-phase nitric acid (HNO3) and ammonia (NH3), freshly nucleated particles can grow extremely rapidly, maintaining a high particle number concentration, even in the presence of a high condensation sink. Such high growth rates may explain the high survival probability of freshly formed particles under haze conditions. We identify under what typical urban conditions HNO3 and NH3 can be expected to contribute to particle survival during haze.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures



References
-
- Merikanto J. Spracklen D. V. Mann G. W. Pickering S. J. Carslaw K. S. Impact of nucleation on global CCN. Atmos. Chem. Phys. 2009;9:8601–8616. doi: 10.5194/acp-9-8601-2009. - DOI
-
- Intergovernmental Panel on Climate Change (IPCC), Climate Change 2013: the Physical Science Basis, 2013
-
- Lohmann U. Feichter J. Global indirect aerosol effects: a review. Atmos. Chem. Phys. 2005;5:715–737. doi: 10.5194/acp-5-715-2005. - DOI
-
- Chen G. Wang W.-C. Chen J.-P. Circulation responses to regional aerosol climate forcing in summer over East Asia. Clim. Dyn. 2018;51:3973–3984. doi: 10.1007/s00382-018-4267-3. - DOI
LinkOut - more resources
Full Text Sources
