Ice nucleation imaged with X-ray spectro-microscopy
- PMID: 35694137
- PMCID: PMC9119033
- DOI: 10.1039/d1ea00077b
Ice nucleation imaged with X-ray spectro-microscopy
Abstract
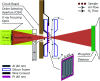
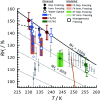
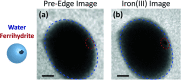
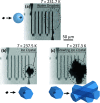
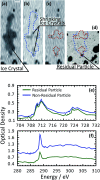
Ice nucleation is one of the most uncertain microphysical processes, as it occurs in various ways and on many types of particles. To overcome this challenge, we present a heterogeneous ice nucleation study on deposition ice nucleation and immersion freezing in a novel cryogenic X-ray experiment with the capability to spectroscopically probe individual ice nucleating and non-ice nucleating particles. Mineral dust type particles composed of either ferrihydrite or feldspar were used and mixed with organic matter of either citric acid or xanthan gum. We observed in situ ice nucleation using scanning transmission X-ray microscopy (STXM) and identified unique organic carbon functionalities and iron oxidation state using near-edge X-ray absorption fine structure (NEXAFS) spectroscopy in the new in situ environmental ice cell, termed the ice nucleation X-ray cell (INXCell). Deposition ice nucleation of ferrihydrite occurred at a relative humidity with respect to ice, RH i, between ∼120-138% and temperatures, T ∼ 232 K. However, we also observed water uptake on ferrihydrite at the same T when deposition ice nucleation did not occur. Although, immersion freezing of ferrihydrite both in pure water droplets and in aqueous citric acid occurred at or slightly below conditions for homogeneous freezing, i.e. the effect of ferrihydrite particles acting as a heterogeneous ice nucleus for immersion freezing was small. Microcline K-rich feldspar mixed with xanthan gum was also used in INXCell experiments. Deposition ice nucleation occurred at conditions when xanthan gum was expected to be highly viscous (glassy). At less viscous conditions, immersion freezing was observed. We extended a model for heterogeneous and homogeneous ice nucleation, named the stochastic freezing model (SFM). It was used to quantify heterogeneous ice nucleation rate coefficients, mimic the competition between homogeneous ice nucleation; water uptake; deposition ice nucleation and immersion freezing, and predict the T and RH i at which ice was observed. The importance of ferrihydrite to act as a heterogeneous ice nucleating particle in the atmosphere using the SFM is discussed.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
We declare no conflict of interest.
Figures

References
-
- Lohmann U. Feichter J. Atmos. Chem. Phys. 2005;5:715–737. doi: 10.5194/acp-5-715-2005. - DOI
-
- Lohmann U. Diehl K. J. Atmos. Sci. 2006;63:968–982. doi: 10.1175/JAS3662.1. - DOI
-
- Mülmenstädt J. Sourdeval O. Delanoë J. Quaas J. Geophys. Res. Lett. 2015;42:6502–6509. doi: 10.1002/2015GL064604. - DOI
-
- Brewer A. W. Q. J. R. Meteorol. Soc. 1949;75:351–363. doi: 10.1002/qj.49707532603. - DOI
-
- Storelvmo T. Annu. Rev. Earth Planet. Sci. 2017;45:199–222. doi: 10.1146/annurev-earth-060115-012240. - DOI
LinkOut - more resources
Full Text Sources
