The Risk Reduction Effect of a Nutritional Intervention With a Partially Hydrolyzed Whey-Based Formula on Cow's Milk Protein Allergy and Atopic Dermatitis in High-Risk Infants Within the First 6 Months of Life: The Allergy Reduction Trial (A.R.T.), a Multicenter Double-Blinded Randomized Controlled Study
- PMID: 35694159
- PMCID: PMC9174747
- DOI: 10.3389/fnut.2022.863599
The Risk Reduction Effect of a Nutritional Intervention With a Partially Hydrolyzed Whey-Based Formula on Cow's Milk Protein Allergy and Atopic Dermatitis in High-Risk Infants Within the First 6 Months of Life: The Allergy Reduction Trial (A.R.T.), a Multicenter Double-Blinded Randomized Controlled Study
Abstract
Background: The role of partially hydrolyzed formulas (pHF) as part of nutritional interventions to prevent the development of allergic manifestations (AM) is questioned, and efficacy of each specific pHF should be substantiated.
Objective: To investigate the risk-reduction effect of a whey-based pHF on the development of cow's milk protein allergy (CMPA) and atopic dermatitis (AD) in infants at high-risk for allergy within the first 6 months of life.
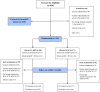
Materials and methods: In a multicenter double-blinded randomized controlled setting, healthy non-exclusively breastfed full-term infants, received either a specific whey-based pHF or a standard cow's milk-based formula (SF) and were clinically assessed for AM at 2, 4, and 6 months of age, supported by the objective scoring tools SCORAD and CoMiSS. CMPA was confirmed by open food challenge. Intention-to-Treat (ITT) and Per-Protocol (PP) analyses were performed.
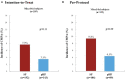
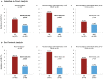
Results: Of 331 randomized subjects (ITT analysis set), 160 received the pHF and 171 the SF. Six (3.8%) infants in the pHF and 12 (7%) in the SF group developed CMPA (p = 0.186). AD incidence was significantly lower in those receiving pHF as compared to SF (10.6% vs. 18.7%, p = 0.024) with a relative risk (RR, 95% CI) of 0.54 (0.32, 0.92), in particular when adjusting for family history of AD [6.5% vs. 27.3%, RR 0.24 (0.07, 0.78), p = 0.018] representing a risk reduction of 76%. The PP analysis showed similar results.
Conclusion: This specific whey-based pHF reduced the risk of AD development, particularly in those with a family history of AD, and tended to reduce the development of CMPA in non-exclusively breastfed infants at high-risk for allergy. The A.R.T. study suggests that this particular pHF may contribute to measures aimed at prevention of allergic manifestations. However, further studies are needed to confirm this risk-reduction effect.
Keywords: allergy prevention; atopic dermatitis; cow milk allergy; high-risk infants; nutritional intervention; partially hydrolyzed formula; randomized controlled trial.
Copyright © 2022 Nicolaou, Pancheva, Karaglani, Sekkidou, Marinova-Achkar, Popova, Tzaki, Kapetanaki, Iacovidou, Boutsikou, Iliodromiti, Papaevangelou, Sardeli, Xepapadaki, Papathoma, Thijs-Verhoeven, Kudla, Ulfman, Schaafsma and Manios.
Conflict of interest statement
NN, PX, and YM have received an honorarium for the speaker's bureau from FrieslandCampina. IT-V, LU, UK, and AS are employees at FrieslandCampina. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous

